Abstract
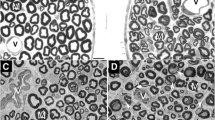
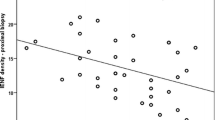
Measurements were made of the thickness of the basal lamina of perineurial cells in the sural nerve in a series of patients with diabetic neuropathy and compared with a group of patients with type I hereditary motor and sensory neuropathy (HMSN) and with organ donor control cases. The thickness was significantly greater in the diabetic patients as compared both with the HMSN cases and the organ donor controls. This was most obvious for the intermediate layers of the perineurium. Perineurial basal laminal thickness was only slightly greater in the HMSN cases than in the organ donor controls and the difference was not statistically significant. The thickening of the perineurial cell basal laminae was compared with the thickening of the basal laminal zone around the endoneurial microvessels. No significant correlation was found either for the diabetic neuropathy or HMSN cases or for the organ donor controls. As had been observed previously, the basal laminal zone around the endoneurial capillaries was of increased thickness both in the diabetic neuropathy and the HMSN cases and, although it was greater for the diabetic neuropathy patients, the difference was not statistically significant. Taken together, these findings indicate that the thickening of the basal lamina of the perineurial cells in a more characteristic feature of diabetic neuropathy than is thickening of the basal laminal zone around the endoneurial capillaries. The results suggest that the causative mechanisms are likely to differ, a conclusion supported by the morphological appearances: the basal laminal thickening around the perineurial cells is uniform, whereas that around the capillaries consists of basal laminal reduplication. Atrophy and necrosis of perineurial cells were observed in patients with diabetic neuropathy but rarely in the cases with HMSN and not in the organ donor cases. This may be similar to the degeneration of endoneurial fibroblasts that has been described as a non-specific finding in neuropathies.
Similar content being viewed by others
References
Baur PS, Stacey TR (1977) The use of PIPES buffer in the fixation of mammalian and marine tissues for electron microscopy. J Microsc 109: 315–327
Beggs JL, Johnson PC, Olafsen AG, Watkins CJ, Tranovnik JH, Koep LJ (1989) Regression of perineurial basement membrane in a human diabetic following isogenic pancreas transplant. Acta Neuropathol 79: 103–112
Behse F, Buchthal F, Carlsen F (1977) Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry 40: 1072–1082
Bell MA, Weddell AGM (1984) A morphometric study of intrafascicular vessels in mammalian sciatic nerve. Muscle Nerve 7: 524–534
Bischoff A (1967) Die Ultrastruktur peripherer Nerven bei der diabetischen Neuropathie. Verh Dtsch Ges Inn Med 72: 1138–1141
Bradley J, Thomas PK, King RHM, Llewelyn JG, Muddle JR, Watkins PJ (1990) Morphometry of endoneurial capillaries in diabetic sensory and autonomic neuropathy. Diabetologia 33: 611–618
Bradley J, Thomas PK, King RHM, Muddle JR (1992) A comparison between the changes in the basal laminae of the perineurium and endoneurial capillaries in diabetic neuropathy and hereditary motor and sensory neuropathy. J Neurol 239 [Suppl 2]: S93
Brownlee M, Spiro RG (1979) Glomerular basement membrane metabolism in the diabetic rat. Diabetes 28: 121–125
Bunge MB, Wood PM, Tynan LB, Bates ML, Sanes JR (1989) Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science 243: 229–231
Fagerberg SE (1959) Diabetic neuropathy: a clinical and histological study on the significance of vascular affections. Acta Med Scand 164 [Suppl 345]: 1–80
Grehl H, Schröder JM (1991) Significance of degenerating endoneurial cells in peripheral neuropathy. Acta Neuropathol 81: 680–685
Guy RJC, Dawson JL, Garrett JR, Laws JW, Thomas PK, Sharma AK, Watkins PJ (1984) Diabetic gastroparesis from autonomic neuropathy: surgical considerations and changes in vagus nerve morphology. J Neurol Neurosurg Psychiatry 47: 686–691
Harding AE, Thomas PK (1980) The clinical features of hereditary motor and sensory neuropathy, types I and II. Brain 103: 259–280
Johnson PC (1983) Thickening of the human dorsal root ganglion cell perineurial cell basement membrane in diabetes mellitus. Muscle Nerve 6: 561–565
Johnson PC, Doll SC (1984) Dermal nerves in human diabetic subjects. Diabetes 33: 244–250
Johnson PC, Brendel K, Meezan E (1981) Human diabetic perineurial basement membrane thickening. Lab Invest 44: 265–270
King RHM, Llewelyn JG, Thomas PK, Gilbey SG, Watkins PJ (1989) Diabetic neuropathy: abnormalities of Schwann cell and perineurial basal laminae. Implications for diabetic vasculopathy. Neuropathol Appl Neurobiol 15: 339–355
Lubec G, Pollak A (1980) Reduced susceptibility of nonenzymatically glycosylated glomerular basement membrane to proteases. Renal Physiol 3: 4–8
Murrah V, Crosson J, Sauk J, (1984) Abnormal binding of negatively charged serum proteins to diabetic basement membranes is largely a systemic phenomenon. Virchows Arch [A] 405: 141–154
Pihlajaniemi T, Myllylä R, Kivirikko KI, Tryggvason K (1982) Effects of streptozotocin diabetes, glucose and insulin on the metabolism of type IV collagen and proteoglycan in murine basement membrane forming EHS tumor tissue. J Biol Chem 257: 14914–14920
Shellswell GB, Restall DJ, Duance VC, Bailey AJ (1979) Identification and differential distribution of collagen types in the central and peripheral nervous system. FEBS Lett 106: 305–308
Sievers J (1971) Basic two-dye stains for epoxy-embedded 0.3–1 μ sections. Stain Technol 46: 195–199
Thomas PK, Jones DG (1967) The cellular response to nerve injury. 2. Regeneration of the perineurium after nerve section. J Anat 101: 45–55
Vital C, Vallat JM, LeBlanc MF, Coquet M (1973) Les neuropathies périphériques du diabète sucré: étude ultrastructurale des 12 cas biopsiés. J Neurol Sci 18: 381–393
Vital C, LeBlanc M, Vallat JM (1974) Étude ultrastructurale du nerf périphérique chez 16 diabétiques sans neuropathie clinique. Comparison avec 16 neuropathies diabétique et 16 neuropathies non diabétiques. Acta Neuropathol (Berl) 30: 63–72
Author information
Authors and Affiliations
Additional information
Supported by the British Diabetic Association and in part by the Medical Research Council and grants from the Stanley Thomas Johnson Foundation, Ciba Geigy Ltd., Basel and Action Research
Rights and permissions
About this article
Cite this article
Bradley, J.L., Thomas, P.K., King, R.H.M. et al. A comparison of perineurial and vascular basal laminal changes in diabetic neuropathy. Acta Neuropathol 88, 426–432 (1994). https://doi.org/10.1007/BF00389494
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00389494