Summary
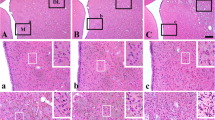
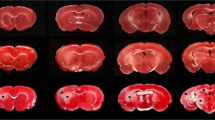
Focal ischemia was induced in rats by occlusion of the middle cerebral artery (MCA). Infarction developed primarily in the basal ganglia. The development, density and distribution of neuronal injury in the cortex adjacent to the infarct were studied from 4 h to 1 year after occlusion of the artery. The brains were perfusion fixed, sub-serially sectioned, and stained with H + E, acid fuchsin/cresyl violet, and ad modum Klüver-Barrera. The number of injured neurons was assessed by direct visual counting. Four hours after the artery occlusion, the infarct was clearly outlined in the corpus striatum, whereas the cortical border became sharp 1 to 2 days after ligation of MCA. After 1 day triangular injured neurons with eosinophilic cytoplasm and pyknotic nuclei were seen outside the infarct. The number of injured neurons at day 1, 2, 3, 4, and 5 was the same, i. e. no evidence for delayed neuronal death was found. Neuron counts at day 1, 4, 10, 17, 27, and 365 were reduced according to the number of acutely injured neurons. Most injured neurons were observed less than 3 mm from the margin of the infarct and the greatest number was found in the cortical layers 2 and 3. The border zone in the medial part of the striatum showed selective neuronal necrosis only in a zone of 200 μm. The fact that the number of injured neurons was constant from day 1 to 5 after artery occlusion indicates that the damage is acute and that a delayed loss of neurons is of minor significance.
Similar content being viewed by others
References
Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94:239–247
Astrup J, Symon L, Branston NM (1977) Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 8:51–57
Auer RN, Wieloch T, Olsson Y, Siesjø BK (1984) The distribution of hypoglycemic damage. Acta Neuropathol (Berl) 64:177–191
Branston NM, Strong AJ, Symon L (1982) Kinetics of resolution of transient increases in extracellular potassium activity: relationship to regional blood flow in the primate cerebral cortex. Neurol Res 4:1–19
Brierley JB, Graham DI (1984) Hypoxia and vascular disorders of the central nervous system. In: Hume Adams J, Corsellis JAN, Duchen LW (eds) Greenfield's neuropathology. Edward Arnold, London, pp 125–207
Bures J, Buresova O (1960) Activation of latent foci of spreading cortical depression in rats. J Neurophysiol 23:225–236
DeGirolami U, Crowell RM, Marcoux FW (1984) Selective necrosis and total necrosis in focal cerebral ischemia. Neuropathologic observations on experimental middle cerebral artery occlusion in the marcaque monkey. J Neuropathol Exp Neurol 43:57–71
Garcia JH, Lossinsky AS, Kauffman FC, Conger KA (1978) Neuronal ischemic injury: light microscopy, ultrastructure and biochemistry. Acta Neuropathol (Berl) 43:85–95
Harris RJ, Symon L, Branston NM, Bayhan M (1981) Changes in extracellular calcium activity in cerebral ischemia. J Cereb Blood Flow Metab 1:203–209
Kalimo H, Rehncrona S, Soderfeldt B, Olsson Y, Siesjø BK (1981) Brain lactic acidosis and ischemic cell damage. 2. Histopathology. J Cereb Blood Flow Metab 1:313–327
Kirino T, Sano K (1984) Fine structural nature of delayed neuronal death following ischemia in the gerbil hippocampus. Acta Neuropathol (Berl) 62:209–218
Lassen NA, Strong AJ, Mies G, Astrup J (1984) Ischemic penumbra results in incomplete infarction: is the sleeping beaty dead. Stroke 14:755–758
Leao AAP (1944) Spreading depression of activity in the cerebral cortex. J Neurophysiol 7:359–390
Leao AAP (1951) The slow voltage variations of cortical spreading depression of activity. Electroencephalogr Clin Neurophysiol 3:315–321
Mies G, Auer LM, Ebhardt G, Traupe H, Heiss W-D (1983) Flow and neuronal density in tissue surrounding chronic infarction. Stroke 14:22–27
Nedergaard M, Astrup J (1986) Infarct rim; effect of hyperglycemia on direct current potential and [14C] 2-deoxyglucose phosphorylation. J Cereb Blood Flow Metab 6:607–615
Nedergaard M, Astrup J, Klinken L (1984) Cell density and cortex thickness in the border zone surrounding old infarct in the human brain. Stroke 15:1033–1039
Nedergaard M, Gjedde A, Diemer NH (1986) Focal ischemia of the rat brain: autoradiographic determination of cerebral glucose utilization, glucose content, and blood flow. J Cereb Blood Flow Metab 6:414–424
Nedergaard M, Vorstrup S, Astrup J (1987) Cell density in the border zone surrounding small old brain infarcts in man. Stroke 17 (in press)
Nicholson C, Kraig RP (1981) The behavior of extracellular ions during spreading depression. In: Zeuthen T (ed) The application of ion-selective microelectrodes. Elsevier/North-Holland Biomedica, Amsterdam, pp 217–238
Siesjø BK (1984) Cerebral circulation and metabolism. J Neurosurg 60:883–908
Skinhøj E, Paulson OB (1970) The mechanism of action of aminophylline upon cerebral vascular disorders. Acta Neurol Scand 46:129–140
Small JV (1968) Measurements of sections thickness. In: Bocciarelly DS (ed) Proceedings 4 th European Congress on Electron Microscopy, vol. 1. Tipografia Poliglotta Vaticana, Roma, p 609
Smith ML, Auer RN, Siesjø BK (1984) The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol (Berl) 64:319–332
Sramka M, Brozek G, Bures J, Nadvornik P (1977) Functional ablation by spreading depression: possible use in human stereotactic neurosurgery. Appl Neurophysiol 40:48–61
Strong AJ, Tomlinson BE, Venables GS, Gibson G, Hardy JA (1983a) The cortical ischaemic penumbra associated with occlusion of the middle cerebral artery in the cat. 2. Studies of histopathology, water content, and in vitro neurotransmitter uptake. J Cereb Blood Flow Metab 3:97–108
Strong, AJ, Venables GS, Gibson G (1983b) The cortical ischaemic penumbra associated with occlusion of the middle cerebral artery in the rat: topography of changes in blood flow, potassium ion activity, and EEG. J Cereb Blood Flow Metab 3:86–96
Symon L, Brierley J (1976) Morphological changes in cerebral blood vessels in chronic ischemic infarction: flow correlation obtained by the hydrogen clearance method. In: Cervos-Navarro J (ed) The cerebral vessel wall. Raven Press, New York, pp 165–174
Tamura A, Graham DI, McCulloch J, Teasdale GM (1981) Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metabol 1:53–60
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nedergaard, M. Neuronal injury in the infarct border: a neuropathological study in the rat. Acta Neuropathol 73, 267–274 (1987). https://doi.org/10.1007/BF00686621
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686621