Summary
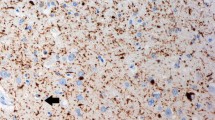
Mapping of striatal and diencephalic plaque distribution was conducted in 25 cases of dementia of the Alzheimer type. This analysis was carried out by fluorescence microscopy of paraffinembedded tissue sections treated with Thioflavine S as fluorochrome. Consistent differences in plaque morphology and density between nuclei and fiber tracts were observed. Striatal and pallidal distribution was uneven, with plaque aggregation near and within certain fiber tracts: capsules, medullary laminae, and radial fasciculi. Diencephalic plaques showed also preferred aggregation near and within fiber tracts and within the intralaminar nuclei.
The different subcortical plaque morphologies observed according to the nuclear or fiber tract location of the amyloid plaque, indicates that the peripheral (“halo”) portion of the plaque is determined by the neuropil response to the primary event: the amyloid deposit.
No correlation was observed between the distribution of plaques and any particular neurotransmitter system. In that respect, plaques were present within the nucleus basalis. Neurofibrillary tangle distribution was also seen to be dissociated from plaque distribution.
Similar content being viewed by others
References
Alzheimer JA (1907) Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiatr 64: 146–148
Beck E, Daniel PM (1979) Kuru and Creutzfeld--Jacob disease: neuropathological lesions and their significance. In: Prusiner SB, Hadlow WJ (eds) Slow transmissible diseases of the nervous system, vol 1 Academic Press, New York, pp 253–285
Blessed G, Tomlinson BE, Roth M (1968) The association between quantitative measurements of dementia and of senile changes in the cerebral gray matter of elderly subjects. Brit J Psychiatry 114: 797–811
Boellard JW, Schlote W (1980) Subakute spongiforme Encephalopathie mit multipler Plaquebildung. Acta Neuropathol (Berl) 49: 205–212
Bouman L (1934) Senile plaques. Brain 57: 128–142
Bouman L, Bok ST (1923) Senile plaques in Corpus striatum. Z Ges Neurol Psychiatr 85: 164–169
Braak H, Braak E (1982) Neuronal types in the claustrum of man. Anat Embryol 163: 447–460
Braunmühl AV (1957) Alterserkrankungen des Zentralnervensystems, senile Involution, senile Demenz, Alzheimer'sche Krankheit. In: Lubarsch O, Henke F, Rossle R (eds) Handbuch der speziellen pathologischen Anatomie und Histologie, vol 13. Springer, Berlin, pp 337–539
Brockhaus H (1940) Die Cyto- und Myeloarchitektonik des Cortex claustralis und des Claustrum beim Menschen. J Psychol Neurol 49: 249–348
Bruce ME, Fraser H (1975) Amyloid plaques in the brains of mice infected with scrapie, morphological variation and staining properties. Neuropathol Appl Neurobiol 1: 189–202
Bruce EM, Fraser H (1981) Effect of route of infection on the frequency and distribution of cerebral amyloid plaques in scrapie mice. Neuropathol Appl Neurobiol 7: 289–298
Burger PC, Vogel FS (1973) The development of the pathologic changes of Alzheimer's disease and senile dementia in patients with Down's syndrome. Am J Pathol 73: 457–476
Carey RG, Bear MF, Diamond IT (1980) The laminar organization of the reciprocal projections between the claustrum and striate cortex in the tree shrew,Tupaia glis. Brain Res 184: 193–198
Chou SM, Martin JD (1971) Kuru plaques in a case of Creutzfeldt-Jakob disease. Acta Neuropathol (Berl) 17: 150–155
Constantinidis J, Tissor R (1979) Plaques seniles, degenerescences neurofibrillaires et autres lesions cérébrales associées. Arch Swiss Neurol Neurochir Psychiatry 124: 317–333
Dayan AD (1970a) Quantitative histological studies on the aged human brain. I. Senile plaques and neurofibrillary tangles in “normal” patients. Acta Neuropathol (Berl) 16: 85–94
Dayan AD (1970b) Quantitative histological studies on the aged human brain. II. Senile plaques and neurofibrillary tangles in senile dementia. Acta Neuropathol (Berl) 16: 95–102
Divry P (1935) Des lésions de l'infundibulum dans démence sénile. J Belge Neurol 35: 591–599
Druga R (1982) Claustro-neocortical connections in the cat and rat demonstrated by HRP tracing technique. J Hirnforsch 23: 191–202
Ferraro A (1931) The origin and formation of senile plaques. Arch Neurol Psychiatry 25: 1042–1062
Fuller SC (1911) A study of the miliary plaques found in brains of the aged. Am J Insanity 48: 147–219
Goldman PS, Nauta WJH (1977) An intricately patterned prefrontocaudate protection in the rhesus monkey. J Comp Neurol 171: 363–386
Gorry JD (1963) Studies on the comparative anatomy of the ganglion basale of Meynert. Acta Anat 55: 51–104
Graybiel AM, Ragsdale CW Jr (1979) Fiber connections of the basal ganglia. Prog Brain Res 51: 237–238
Graybiel AM, Ragsdale CW, Jr (1980) Clumping of acetylcholinesterase activity in the striatum of the human fetus and young infant. Proc Natl Acad Sci USA 77: 1214–1218
Graybiel AM, Ragsdale CW Jr, Yoneoka ES, Elde RP (1981) An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetycholinesterase staining. Neuroscience 6: 377–397
Grünthal E (1926) Über die Alzheimersche Krankheit. Eine histopathologisch-klinische Studie. Z Ges Neurol Psychiatr 101: 128–157
Heimer L, Wilson RD (1975) The subcortical projections of the allocortex; similarities in the neural associations of the hippocampus, the piriform cortex and the neocortex. In: Santini M (ed) Golgi centennial symposium. Raven Press, New York, pp 177–193
Hirano A, Ghatak NR, Johnson AB, Pathnow MJ, Gomorei AJ (1972) Argentophilic plaques in Creutzfeldt-Jakob disease. Arch Neurol 26: 530–542
Hooper MW, Vogel FS (1976) The limbic system in Alzheimer's disease. Am J Pathol 85: 1–20
Jamada M, Mehraein P (1968) Verteilungsmuster der senilen Veränderungen im Gehirn. Arch Psychiatr Nervenkr 211: 308–324
Jervis GA (1948) Early senile dementia in mongoloid idiocy. Am J Psychiatry 105: 102–106
Jones EG, Leavitt RY (1974) Retrograde axonal transport and the demonstration of non-specific profections to cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol 154: 349–378
Jones EG, Coulter JD, Burton H, Porter R (1977) Cell of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol 173:53–80
Kaitz SS, Robertson RT (1981) Thalamic connections with limbic cortex. II. Corticothalamic projections. J Comp Neurol 195:527–545
Kelenyi G (1967) On the histochemistry of azo group-free thiazole dyes. J Histochem Cytochem 13:172–180
Kimberlin RH, Walker CA (1982) Pathogenesis of mouse scrapie: patterns of agent replication in different parts of the CNS following intraperitoneal infection. J R Soc Med 35:618–624
Klatzo V, Gajdusek DC, Zigas V (1959) Pathology of kuru. Lab Invest 8:799–847
Kunzle H (1974) Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study inMacaca fascicularis. Brain Res 88:195–209
Lang W, Henke H (1982) Cholinergic enzymes and muscarinic cholinergic receptors in senile dementia of Alzheimer type (SDAT). IXth Int Cong Neuropathol, Vienna, Austria
LeVay S, Sherk H (1981) The visual claustrum of the cat. I. Structure and connections. J Neurosci 1:956–980
Lhermitte J, Trelles JO (1934) Plaques seniles de l'infundibulum. Rev Neurol (Paris) 41:547–550
Lillie RD (1977) HJ Conn's Biological stains. Williams and Wilkins, Baltimore, pp 369–371
Macchi G, Bentivoglio M, Minciacchi D, Molinari M (1981) The organization of the claustroneocortical projections in the cat studied by means of the HRP retrograde axonal transport. J Comp Neurol 195:681–695
Malamud N (1972) Neuropathology of organic brain syndromes associated with aging. In: Gaitz CM (ed) Aging and the brain. Plenum Press, New York, pp 63–87
Marchand R, Poirer LJ, Parent A (1979) Cytohistochemical study of the primate basal ganglia and substantia nigra. Adv Neurol 24:13–24
Master CL, Gadjusek DC, Gibbs CJ (1980) The Gerstmann-Sträussler syndrome and the various forms of amyloid plaques which occur in the transmissible spongiform encephalopathies. J Neuropathol Exp Neurol 39:374
Moretz RC, Wisniewski HM, Lossinsky AS (1983) Pathogenesis of neuritic and amyloid plaques in scrapie — Ultrastructural study of early changes in the cortical neuropil. In: Samuel D (ed) Aging of the brain, vol 22. Raven Press, New York, pp 61–79
Morimatsu M, Hirai S, Muramatsu A, Yoshikawa M (1974) Senile degenerative brain lesions and dementia. J Am Geriatr Soc 23:390–406
Narkiewicz O (1964) Degenerations in the claustrum after regional neocortical ablations in the cat. J Comp Neurol 123:335–356
Nobin A, Bjørklund A (1973) Topography of the monoamine neuron systems in the human brain as revealed in fetuses. Acta Physiol Scand [Suppl 388]88:1–40
Pert CB, Kuhar MJ, Synder SH (1976) Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci USA 3:3729–3733
Powers JM, Schlaepfer WW, Willingham MC, Hall BJ (1981) An immunoperoxidase study of senile cerebral amyloidosis with pathogenetic considerations. J Neuropathol Exp Neurol 40:592–612
Riley HA (1960) An atlas of the basal ganglia, brainstem and spinal cord, 2nd edn, Hafner, New York
Robertson RT, Kaitz SS (1981) Thalamic connections with limbic cortex. I. Thalamocortical projections. J Comp Neurol 195:501–525
Roth M, Tomlinson BE, Blessed G (1966) Correlation between scores for dementia and counts of “senile plaques” in cerebral gray matter of elderly patients. Nature 209:109–110
Rudelli RD, Ambler MW, Wisniewski HM (1984) Pattern of distribution of Alzheimer plaques in brain stem. J Neuropathol Exp Neurol 43:306
Sanides D, Buchholtz CS (1979) Identification of the projection from the visual cortex to the claustrum by anterograde axonal transport in the cat. Exp Brain Res 35:197–200
Schwartz P (1970) Amyloidosis. Cause and manifestation of senile deterioration. Thomas, Springfield, IL, pp 303–304
Schwartz P, Kurucz J, Furucz A (1964) Recent observations on senile cerebral changes and their pathogenesis. J Am Geriatr Soc 12:908–922
Simchowicz T (1924) Sur la significations des plaques seniles et sur la formule senile de l'écorce cérébrale. Rev Neurol (Paris) 1:221–227
Simma K (1951) Über Thalamusveränderungen bei seniler Demenz und bei der Alzheimer'schen Krankheit. Psychiat Neurol 122:156–178
Strauss EE (1956) Clinopathologic report of Alzheimer's disease. J Neuropathol Exp Neurol 15:233–235
Struble RG, Cork LC, Whitehouse PJ, Price DL (1982) Cholinergic innervation in neuritic plaques. Science 216: 413–415
Swanson LW, Cowan WM (1975) A note on the connections and development of thenucleus accumbens. Brain Res 92:324–330
Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS (1981) Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol 10:184–192
Tomlinson BE, Blessed G, Roth M (1968) Observations on the brains of non-demented old people. J Neurol Sci 7:331–356
Tomlinson BE, Blessed G, Roth M (1970) Observations on the brains of deviated old people. J Neurol Sci 11:205–242
Van Buren JM, Borke RC (1972) Variations and connections of the human thalamus. Springer, Berlin Heidelberg New York
Vassar PS, Culling CFA (1959) Fluorescent stains with special reference to amyloid and connective tissue. Arch Pathol 68:487–498
Walker AE (1966) Internal structure and afferent-efferent relations of the thalamus. In: Purpura DP, Yahr MD (eds) The thalamus. Columbia University Press, New York London, pp 1–12
Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR (1981) Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol 10:122–126
Wilcock GK, Esiri MM (1982) Plaques tangles and dementia. J Neurol Sci 56:343–356
Wildi E, Dago-Akribi A (1968) Alterations cérébrale chez l'homme age. Bull Schweiz Akad Med Wiss 24:107–132
Wildi E, Linder A, Costoulas G (1964) Etude statistique des alterations dégénératives cérébrales apparaissant au cours du vieillissement. Psychiat Neurol 148:41–68
Wisniewski HM, Terry RD (1973a) Morphology of the aging brain, human and animal. Prog Brain Res 40:167–186
Wisniewki HM, Terry RD (1973b) Reexamination of the pathogenesis of the senile plaque. In: Zimmerman HM (ed) Progress in neuropathology, vol. 2 Grune & Stratton, New York, pp 1–25
Wisniewski HM, Merz GS (1983) Neuritic and amyloid plaques in senile dementia of the Alzheimer type. In: Katzman R (ed) Banbury Report 15: Biological aspects of Alzheimer's disease. Cold Spring Harbor Laboratory, Cold Spring Harbor, pp 145–153
Wisniewski HM, Johnson AB, Raine CS, Kay WS, Terry RD (1970) Senile plaques and cerebral amyloidosis in aged dogs. A histochemical and ultrastructural study. Lab Invest 23:287–296
Wisniewski HM, Ghetti B, Terry RD (1973) Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol 32:566–584
Wisniewki HM, Lossinsky AS, Moretz RC, Vorbrodt AW (1981a) Neuritic plaque formation and blood brain barrier changes in scrapie. J Neuropathol Exp Neurol 40:342
Wisniewski HM, Moretz RC, Lossinsky AS (1981b) Evidence for indication of localized amyloid deposits and neuritic plaques by an infectious agent. Ann Neurol 10:517–522
Wisniewski KE, Wisniewski HM, Wen GY (1983) Plaques, tangles, and dementia in Down syndroem. J Neuropathol Exp Neurol 42:340
Wisniewski KE, Dalton AJ, McLachlan DS, Wen GY, Wisniewsky HM (1984) Alzheimer disease in Down syndrome — prospective studies. Neurology (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rudelli, R.D., Ambler, M.W. & Wisniewski, H.M. Morphology and distribution of Alzheimer neuritic (senile) and amyloid plaques in striatum and diencephalon. Acta Neuropathol 64, 273–281 (1984). https://doi.org/10.1007/BF00690393
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00690393