Abstract
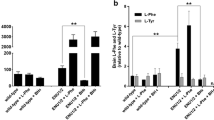
Experimental hyperphenylalaninemia has been induced in 5-day-old chicks by dietary treatments with phenylalanine and α-methylphenylalanine. An increase of nearly 8-fold in plasma Phe/Tyr ratio was found after 4 days of supplementation the standard diet with 5% phenylalanine plus 0.4% α-methylphenylalanine. The increase in this ratio was about 13-fold after 9 days of the same treatment. Similar results were observed in brain and liver, although the increases were smaller than those found in plasma. Total body, brain and liver weight decreased after 9 days of treatment. Phenylalanine plus α-methylphenylalanine administration to 5-day-old chicks produced a significant decrease in the 3-hydroxy-3-methylglutary-CoA reductase and mevalonate-5-pyrophosphate decarboxylase specific activities from both brain and liver. These results demonstrated for the first time that experimental hyperphenylalaninemia inhibited different enzyme activites directly implicated in the regulation of cholesterogenesis. Therefore, a reduced cholesterol synthesis in brain may evidenciate the theory of an impaired myelination leading to mental retardation in phenylketonuria patients.
Similar content being viewed by others
References
Crome, L., and Pare, C. M. B. 1960. Phenylketonuria. A review and a report of the pathological findings in four cases. J. Ment. Sci. 106:862–883.
Shah, S. N., Peterson, N. A., and McKean, C. M. 1972. Lipid composition of human cerebral white matter and myelin in phenylketonuria. J. Neurochem. 19:2369–2376.
Shah, S. N., Peterson, N. A., and McKean, C. M. 1972. Impaired myelin formation in experimental hyperphenylalaninemia. J. Neurochem. 19:479–485.
Menkes, J. H. 1967. The pathogenesis of mental retardation in phenylketonuria and other inborn errors of amino acid metabolism. Pediatrics 39:297–308.
Crome, K., Tymms, V., and Woolf, L. I. 1962. A chemical investigation of the defects of myelination in phenylketonuria. J. Neurol. Neurosurg. Psychiat. 25:143–148.
Gerstl, B., Malamud, N., Eng, L. F., and Hayman, R. B. 1967. Lipid alteration in human brains in phenylketonuria. Neurology 17:51–57.
Foote, J. L., Allen, R. J., and Agranoff, B. W. 1965. Fatty acids in esters and cerebrosides of human brain in phenylketonuria. J. Lipid Res. 6:518–524.
Siperstein, M. D. 1970. Regulation of cholesterol synthesis in normal and malignant tissues. Curr. Top. Cell. Reg. 2:65–100.
Schroepfer, G. J., Jr. 1981. Sterol biosynthesis. Annu. Rev. Biochem. 50:581–621.
Ramachandran, C. K., and Shah, S. N. 1976. Decarboxylation of mevalonate pyrophosphate is one rate-limiting step in hepatic cholesterol synthesis in suckling and weaned rats. Biochem. Biophys. Res. Commun. 69:42–47.
Jabalquinto, A. M., and Cardemil, E. 1980. Secondary regulatory sites in rat liver cholesterol synthesis: Role of 5-pyrophosphomevalonate decarboxylase. Lipids 15:196–199.
Marco, C., Gonzalez-Pacanowska, D., Linares, A., and Garcia-Peregrin, E. 1983. Relationship between changes in free cholesterol and pyrophosphomevalonate decarboxylase activity during myelination. Neurochem. Res. 8:711–721.
Alejandre, M. J., Marco, C., Gonzalez-Pacanowska, D., Segovia, J. L., and Garcia-Peregrin, E. 1983. Inhibition of brain pyrophosphomevalonate decarboxylase in experimental hyperphenylalaninemia induced during myelination. IRCS Med. Sci. 11:744–745.
Marco, C., Alejandre, M. J., Gonzalez-Pacanowska, D., Segovia, J. L., and Garcia-Peregrin, E. 1985. Evolution of 3-hydroxy-3-methylglutaryl-CoA reductase and microsomal membrane fluidity throughout chick embryo development. Int. J. Biochem. 17:271–274.
Alejandre, M. J., Ramirez, H., Suarez, M. D., and Garcia-Peregrin E. 1981. Different developmental patterns of 3-hydroxy-3-methylglutaryl-CoA reductase in chick tissues according to their role in cholesterogenesis. Biol. Neonate 40:232–236.
Gonzalez-Pacanowska, D., Marco, C., Garcia-Martinez, J., and Garcia-Peregrin, E. 1984. Changes in chick liver and brain mevalonate kinase, mevalonate-5-phosphate kinase and mevalonate-5-pyrophosphate decarboxylase during development. Int. J. Biochem. 16:845–847.
Aguilera, J. A., Linares, A., Arce, V., and Garcia-Peregrin, E. 1984. Effect of dietary cholesterol on mevalonate metabolism by sterol and nonsterol pathways. Biochem. Biophys. Res. Commun. 122:945–948.
Ramirez, H., Alejandre, M. J., Segovia, J. L., and Garcia-Peregrin, E. 1981. Different thermal behavior of neonatal hepatic and cerebral 3-hydroxy-3-methylglutaryl-CoA reductase. Lipids 16:552–554.
Gonzalez-Pacanowska, D., Garcia-Martinez, J., and Garcia-Peregrin, E. 1981. Brain phosphorylation and decarboxylation of mevalonic acid in the neonatal chick. Enzyme 26:136–143.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Clarke, J. T. R., and Lowden, J. A. 1969. Hyperphenylalaninemia: effect on the developing rat brain. Can. J. Biochem. 47:291–294.
Johnson, R. C., and Shah, S. N. 1973. Effect of hyperphenylalaninemia on fatty acid composition of lipids of rat brain myelin. J. Neurochem. 21:1225–1240.
Greengard, O., Yoss, M. S., and Del Valle, J. A. 1976. α-Methylphenylalanine, a new inducer of chronic hyperphenylalaninemia in suckling rats. Science 192:1007–1009.
Marco, C., Gonzalez-Pacanowska, D., Zafra, M. F., Segovia, J. L., and Garcia-Peregrin, E. 1984. Induction of experimental phenylketinuria-like conditions in chick embryo. Effect on amino acid concentration in brain, liver and plasma. Neurochem. Int. 6:485–489.
Alejandre, M. J., Marco, C., Ramirez, H., Segovia, J. L., and Garcia-Peregrin, E. 1984. Lipid composition of brain myelin from normal and hyperphenylalaninemic chick embryos. Comp. Biochem. Physiol. 77B:329–332.
Aguilera, J. A., Linares, A., Marco, C., Arce, V., and Garcia-Peregrin, E. 1984. Postnatal development of the sterol and nonsterol mevalonate metabolism in chick liver and kidneys. Effect of cholesterol feeding. Ann. Nutr. Metab. 28:342–349.
Nordyke, E. L., and Roach, M. K. 1974. Effect of hyperphenylalaninemia on amino acid metabolism and compartmentation in neonatal rat brain. Brain Res. 67:479–488.
Johnson, R. C., and Shah, S. N. 1980. Effects of α-methylphenylalanine plus phenylalanine treatment during development on myelin in rat brain. Neurochem. Res. 5:709–718.
Lane, J. D., and Neuhoff, V. 1980. Phenylketonuria. Clinical and experimental considerations revealed by the use of animal models. Naturwissenschaften 67:227–233.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Castillo, M., Zafra, M.F. & Garcia-Peregrin, E. Inhibition of brain and liver 3-hydroxy-3-methylglutaryl-CoA reductase and mevalonate-5-pyrophosphate decarboxylase in experimental hyperphenylalaninemia. Neurochem Res 13, 551–555 (1988). https://doi.org/10.1007/BF00973296
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00973296