Abstract
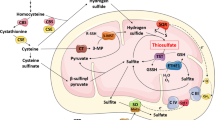
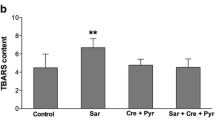
The effect of depletion of reduced glutathione (GSH) on brain mitochondrial function and N-acetyl aspartate concentration has been investigated. Using pre-weanling rats, GSH was depleted by L-buthionine sulfoximine administration for up to 10 days. In both whole brain homogenates and purified mitochondrial preparations complex IV (cytochrome c oxidase) activity was decreased, by up to 27%, as a result of this treatment. In addition, after 10 days of GSH depletion, citrate synthase activity was significantly reduced, by 18%, in the purified mitochondrial preparations, but not in whole brain homogenates, suggesting increased leakiness of the mitochondrial membrane. The whole brain N-acetyl aspartate concentration was also significantly depleted at this time point, by 11%. It is concluded that brain GSH is important for the maintenance of optimum mitochondrial function and that prolonged depletion leads also to loss of neuronal integrity. The relevance of these findings to Parkinson's disease and the inborn errors of glutathione mtabolism are also discussed.
Similar content being viewed by others
References
DiMonte, D. A., Chan, P., and Sandy, M. S. 1992. Glutathione in Parkinson's Disease: A link between oxidative stress and mitochondrial damage? Ann. Neurol. 32:S111–115.
Meister, A., and Larsson, A. 1989. Glutathione synthetase deficiency and other disorders of the γ-glutamyl cycle. Pages 855–868, in Scriver, C. R., Beaudet, A. L., Sly, W. S., and Valle D. (eds), The Metabolic Basis of Inherited Disease, McGraw-Hill, New York.
Jenner, P., Dexter, D. T., Sian, J., Shapira, A. H. V., and Marsden, C. D. 1992. Oxidative stress as a cause of nigral cell death in Parkinson's Disease and incidental Lewy body disease. Ann. Neurol. 32:S82–87.
Boveris, A., and Chance, B. 1973. The mitochondrial generation of hydrogen peroxide. Biochem. J. 134:707–716.
Shan, X., Finkelstien, M., Jones, D. P., and Anders, M. W. 1993. Experimental manipulation of mitochondrial glutathione concentration. Pages 227–234, in Lash L. H., and Jones D. P. (eds), Mitochondrial Dysfunction, Methods in Toxicology Volume 2, Academic Press, London.
Mårtensson, J., Lai, J. C. K., and Meister, A. 1990. A high affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc. Natl. Acad. Sci. USA 87: 7185–7189.
Zhang, Y., Marcillat, O., Giulivi, C., Ernster, L., and Davies, K. J. A. 1990. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 265: 16330–16336.
Schapira, A. H. V., Cooper, J. M., Dexter, D., Clark, J. B., Jenner, P., and Marsden, C. D. 1990. Mitochondrial complex I deficiency in Parkinson's Disease. J. Neurochem. 54:823–827.
Meister, A. 1984. New aspects of glutathione biochemistry and transport: Selective alteration of glutathione metabolism. Fed. Proc. 43:3031–3041.
Masukawa, T., Sai, M., and Tochino, Y 1989. Methods of depleting brain glutathione. Life Sci. 44:417–424.
Jain, A., Mårtensson, J., Stole, E., Auld, P. A. M., and Meister, A. 1991. Glautathione deficiency leads to mitochondrial damage in brain. Proc. Natl. Acad. Sci. USA. 88:1913–1917.
Benzi, G., Curti, D., Pastoris, C., Marzatico, F., Villa, R. F., and Dagani, F. 1991. Sequential damage in mitochondrial complexes by peroxidative stress. Neurochem. Res. 16:1295–1302.
Slivka, A., Mytilineou, C., and Cohen, G. 1987. Histochemical evaluation of glutathione in brain. Brain Res. 409:275–284.
Raps, S. P., Lai, J. C. K., Hertz, L., and Cooper, A. J. L. 1989. Glutathione is present in high concentrations in cultured astrocytes but not cultured neurons. Brain. Res. 493:398–401.
Ratan, R. R., Murphy, T. H., and Baraban, J. M. 1994. Oxidative stress induces apoptosis in embryonic cortical neurons. J. Neurochem. 62:376–379.
Koller, K. J., Zaczek, R., and Coyle, J. T. 1984. N-acetyl-aspartyl-glutamate: regional levels in rat brain and the effect of brain lesions as determined by a new HPLC method. J. Neurochem. 43: 1136–1142.
Moffett, J. R., Namboodiri, M. A. A., Cangro, C. B., and Neale, J. H. 1991. Immunohistochemical localization of N-acetylaspartate in rat brain. Neuroreport 2:131–134.
Simmons, M. L., Frondoza, C. G., and Coyle, J. T. 1991. Immunocytochemical localisation of N-acetyl aspartate with monoclonal antibodies. Neurosci. 45:37–45.
Urenjak, J., Williams, S. R., Gadian, D. G., and Noble, M. 1992. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocytes and immature oligodendrocytes in vitro. J. Neurochem. 59:55–61.
Calvin, H. I., Medvedovsky, C., and Worgul, B. V. 1986. Near total glutathione depletion and age specific cataracts induced by buthionine sulfoximine. Science 233:553–555.
Lai, J. C. K., and Clark, J. B. 1979. Preparation of synaptic and non-synaptic mitochondria from mammalian brain. Methods Enzymol. 55:51–60.
Wharton, D. C., and Tzagoloff, A. 1967. Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 10:245–250.
Shepherd, J. A., and Garland, P. B. 1969. Citrate synthase from rat liver. Methods Enzymol. 13:11–19.
Ragan, C. I., Wilson, M. T., Darley-Usmar, V. M., and P. N. Lowe. 1987. Sub-fractionation of mitochondria and isolation of the proteins of oxidative phosphorylation. Pages 79–112, in Darley-Usmar, V. M., Rickwood, D., and Wilson, M. T. (eds), Mitochondria a Practical Approach, IRL Press, Oxford.
Gornall, A. S., Bardwill, C. J., and David, M. M. 1949. Determination of serum proteins by theans of the biuret reaction. J. Biol. Chem. 177:751–766.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193:265–275.
Riederer, P., Sofic, E., Rausch, W.-D., Schmidt, B., Reynolds, G. P., Jellinger, K., and Youdim, M. B. H. 1989. Transition metals, ferritin, glutathione and ascorbic acid in Parkinsonian brains. J. Neurochem. 52:512–520.
Slivka, A., Spina, M. B., Calvin, H. I., and Cohen, G. 1988. Depletion of brain glutathione in preweanling mice by L-buthionine sulfoximne. J. Neurochem. 50:1391–1393.
Land, J. M., Booth, R. F. G., Berger, R., and Clark, J. B. 1977. Development of mitochondrial energy metabolism in the rat brain. Biochem. J. 164:339–348.
Manning, B. W., and Franklin, M. R. 1990. Induction of rat UDP-glucuronosyltransferase and glutathione S-transferase activities by L-buthionine-S,R-sulfoximine without induction of cytochrome P-450. Toxicol. 65:149–159.
Sokol, R. J., Deveraux, M. W., O'Brien, K., Khandwala, R. A., and Loehr, J. P. 1993. Abnormal hepatic mitochondrial respiration and cytochrome c oxidase activity in rats with long term copper overload. Gastroent. 105:178–187.
Hartley, A., Cooper, J. M., and Shapira, A. H. V. 1993. Iron induced oxidative stress and mitochondrial dysfunction: relevance to Parkinson's disease. Brain Res. 67:349–353.
Thomas, P. K., Cooper, J. M., King, R. H. M., Workman, J. M., Schapira, A. H. V., Goss-Sampson, M. A., and Muller, D. P. R. 1993. Myopathy in vitamin E deficient rats: muscle fibre necrosis associated with disturbances of mitochondrial function. J. Anat. 183:451–461.
Soussi, B., Idstrom, J-P., Schersten, T., and Bylund-Fellenius, A-C. 1990. Cytochrome c oxidase and cardiolipin alterations in response to skeletal muscle ischaemia and reperfusion. Acta. Physiol. Scand. 138:107–114.
Halestrap, A. P., Griffiths, E. J., and Connern, C. P. 1993. Mitochondrial calcium handling and oxidative stress. Biochem. Soc. Trans. 21:353–362.
Reichman, N., Porteous, C. M., and Murphy, M. P. 1994. Cyclosporin A blocks 6-hydroxydopamine-induced efflux of Ca2+ from mitochondria without inactivating the mitochondrial innermembrane pore. Biochem. J. 297:151–155.
Veitch, K., Hombroeckx, A., Caucheteux, D., Pouleur, H., and Hue L. 1992. Global ischaemia induces a biphasic response of the mitochondrial respiratory chain. Biochem. J. 281:709–715.
Mårtensson, J., and Meister, A. 1989. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc. Natl. Acad. Sci. USA 86:471–475.
Mårtensson, J., Steinherz, R., Jain, A., and Meister, A. 1989. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc. Natl. Acad. Sci. USA 86: 8727–8731.
Masini, A., Ceccarelli, D., Gallesi, D., Giovannini, F., and Trenti, T. 1994. Lipid hydroperoxide induced mitochondrial dysfunction following acute ethanol intoxication in rats. The critical role for mitochondrial reduced glutathione. Biochem. Pharmacol. 47:217–224.
Patel, T. B., and Clark, J. B. 1979. Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem. J. 184:539–546.
Dipasquale, B., Marini, A. M., and Youle, R. J. 1991. Apoptosis and DNA degradation by 1-methyl-4-phenylpyridinium in neurons. Biochem. Biophys. Res. Commun. 181:1442–1448.
Tallan, H. H. 1957. Studies on the distribution of N-acetyl-L-aspartic acid in brain. J. Biol. Chem. 224:41–45.
Miyake, M., and Kakimoto, Y. 1981. Developmental changes of N-acetyl-L-aspartic acid, N-acetyl-α-aspartylglutamic acid and β-citryl-L-glutamic acid in different brain regions and spinal cords of rat and guinea pig. J. Neurochem. 37:1064–1067.
Bates, T. E., Williams, S. R., Gadian, D. G., Bell, J. D., Small, R. K., and Iles, R. A. 1989. A1H NMR study of cerebral development in the rat. NMR Biomed. 2:225–229.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heales, R., Davies, S.E.C., Bates, T.E. et al. Deoletion of brain glutathione is accompanied by impaired micochondrial function and decreased N-acetyl aspartate concentration. Neurochem Res 20, 31–38 (1995). https://doi.org/10.1007/BF00995149
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00995149