Abstract
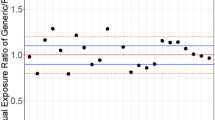
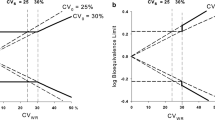
Current procedures for assessing the bioequivatence of two formulations are based on the concept of average bioequivalence. That is, they assess whether the average responses between individuals on the two formulations are similar. Average bioequivalence, however, is not sufficient to guarantee that an individual patient could be expected to respond similarly to the two formulations. To have reasonable assurance that an individual patient could be switched from a therapeutically successful formulation to a different formulation (e.g., a generic substitute) requires a different notion of bioequivalence, which we refer to as individual (or within-subject) bioequivalence. We propose a simple, valid statistical procedure for assessing individual bioequivalence. The decision rule, TIER (Test of Individual Equivalence Ratios), requires the specification of the minimum proportion of subjects in the applicable population for which the two formulations being tested must be bioequivalent (a regulatory decision). The TIER rule is summarized in terms of the minimum number of subjects with bioavailability ratios falling within the specified equivalence interval necessary to be able to claim bioequivalence for given sample size and Type I (α) error. We recommend that the corresponding lower bounds (one-sided confidence intervals) for the proportion of bioequivalent subjects be calculated. TIER is partly motivated by the U.S. FDA's 75/75 Rule (at least 75% of the individual subject bioavailability ratios must be within 75–125%). TIER retains the sensible idea of considering the individual ratios but, unlike the 75/75 rule, is a statistically valid procedure.
Similar content being viewed by others
References
W. J. Westlake. Statistical aspects of comparative bioavailability trials.Biometrics 35:273–280 (1979).
W. J. Westlake. Bioavailability and bioequivalence of pharmaceutical formulations. In K. E. Peace (ed.),Pharmaceutical Statistics for Drug Development, Marcel Dekker, New York, 1988, pp. 329–352.
B. E. Cabana. Assessment of 75/75 rule: FDA viewpoint.J. Pharm. Sci. 72:98–99 (1982).
J. D. Haynes. Statistical simulation study of new proposed uniformity requirement for bioequivalency studies.J. Pharm. Sci. 70:673–675 (1981).
W. J. Conover.Practical Nonparametric Statistics, 2nd ed., Wiley, New York, 1980.
S. Anderson and W. W. Hauck. A new procedure for testing equivalence in comparative bioavailability and other clinical trials.Commun. Statist. A12:2663–2692 (1983).
W. W. Hauck and S. Anderson. A new statistical procedure for testing equivalence in two-graoup comparative bioavailability trials.J. Pharmacokin. Biopharm. 12:83–91 (1984).
C. M. Metzler and D. C. Huang. Statistical methods for bioavailability and bioequivalence.Clin. Res. Practices & Drug Res. Affairs 59:109–132 (1983).
B. Thiyagarajan and T. W. Dobbins. An assessment of the 75/75 rule in bioequivalence. 1987 Proceedings of the Biopharmaceutical Section, American Statistical Association, Alexandria, VA, 1988, pp. 143–148.
Food and Drug Administration. Report by the Bioequivalence Task Force on recommendations from the bioequivalence hearing conducted by the Food and Drug Administration September 29–October 1, 1986 (1988).
D. Clayton and A. Leslie. The bioavailability of erythromycin stearate versus enteric-coated erythromycin base when taken immediately before and after food.J. Int. Med. Res. 9:470–477 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anderson, S., Hauck, W.W. Consideration of individual bioequivalence. Journal of Pharmacokinetics and Biopharmaceutics 18, 259–273 (1990). https://doi.org/10.1007/BF01062202
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01062202