Summary
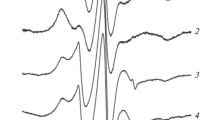
Haemosiderin has been isolated from siderosomes and ferritin from the cytosol of livers of rats iron-loaded by intraperitoneal injections of iron-dextran. Siderosomal haermosiderin, like ferritin, was shown by electron diffraction to contain iron mainly in the form of small particles of ferrihydrite (5Fe2O3 · 9H2O), with average particle diameter of 5.36±1.31 nm (SD), less than that of ferritin iron-cores (6.14±1.18 nm). Mössbauer spectra of both iron-storage complexes are also similar, except that the blocking temperature,T B, for haemosiderin (23 K) is lower than that of ferritin (35 K). These values are consistent with their differences in particle volumes assuming identical magnetic anisotropy constants. Measurements of P/Fe ratios by electron probe microanalysis showed the presence of phosphorus in rat liver haemosiderin, but much of it was lost on extensive dialysis. The presence of peptides reacting with anti-ferritin antisera and the similarities in the structures of their iron components are consistent with the view that rat liver haemosiderin arises by degradation of ferritin polypeptides, but its peptide pattern is different from that found in humanβ-thalassaemia haemosiderin. The blocking temperature, 35 K, for rat liver ferritin is near to that reported, 40 K, for humanβ-thalassaemia spleen ferritin. However, the haemosiderin isolated from this tissue, in contrast to that from rat liver, had aT B higher than that of ferritin. The iron availability of haemosiderins from rat liver and humanβ-thalassaemic spleen to a hydroxypyridinone chelator also differed. That from rat liver was equal to or greater, and that from human spleen was markedly less, than the iron availability from either of the associated ferritins, which were equivalent. The differences in properties of the two types of haemosiderin may reflect their origins from primary or secondary iron overload and differences in the duration of the overload.
Similar content being viewed by others
References
Andrews SC (1986) Studies on ferritin and haemosiderin from rat liver. PhD Thesis, University of Sheffield
Andrews SC, Treffry A, Harrison PM (1987b) Siderosomal ferritin. The missing link between ferritin and haemosideri? Biochem J 245:439–446
Andrews SC, Treffry A, Harrison PM (1987b) A new form ferritin heterogeneity explained. Isolation and identification tion of a nineteen-amino-acid-residue fragment from side rosomal ferritin from rat liver. Biochem J 245:447–453
Arosio P, Adelman TG, Drysdale JW (1978) On ferritin het ogeneity. Further evidence for heteropolymers. J B Chem 253:4451–4458
Bell SH, Weir MP, Dickson DPE, Gibson JF, Sharp GA, Peters TJ (1984) Mössbauer spectroscopic studies of hum haemosiderin and ferritin. Biochim Biophys Acta 787:227–236
Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC (1984) A rapid sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on western blots. Anal Biochem 136:175–179
Brady MC, Lilley KS, Teffry A, Harrison PM (1987) Mobilization of iron from ferritin, haemosiderin and ferritin iron-cores with synthetic hydroxy-pyridinone chelators in vitro. Abstract, Eight International Conference on Proteins of Iron Transport and Storage, Quebec, Canada, p 26
Cleton MI, Frenkel EJ, De Bruijn WC, Marx JJ (1986) Determination of iron to phosphorus ratios of iron storage compounds in patients with iron overload. A chemical and electron probe X-ray microanalysis. Hepatology 6:848–851
Drysdale JW, Munro HN (1965) Small-scale isolation of ferritin for the assay of incorporation of14C-labelled amino acids. Biochem J 95:851–857
Fischbach FA, Gregory DW, Harrison PM, Hoy TG, Williams JM (1971) On the structure of haemosiderin and its relationship to ferritin. J Ultrastruct Res 37:495–503
Fleming J, Joshi JG (1987) Ferritin: isolation of aluminiumferritin complex from brain. Proc Natl Acad Sci USA 84:7866–7870
Fling SP, Gregerson DS (1986) Peptide and molecular weight determination by electrophoresis using a high-molarity tris-buffer system without urea. Anal Biochem 155:83–88
Ford GC, Harrison PM, Rice DW, Smith JMA, Treffry A, White JL, Yariv J (1984) Ferritin: design and formation of an iron-storage molecule. Phil Trans R Soc Lond B 304:551–565
Harrison PM, Andrews SC, Ford GC, Smith JMA, Treffry A, White JL (1987) Ferritin and bacterioferritin: iron sequestering molecules from man to microbe. In: Winkelman G, Helm D van der, Neilands JB (eds) Iron transport in microbes, plants and animals. VCH Verlagsgesellschaft, Weinheim, pp 445–475
Iancu TC (1983) Iron overload. Mol Aspects Med 6:1–100
Liu FT, Zinnecker M, Hamaska T, Katz DH (1979) New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer ofD-amino acids and immunochemical characterization of such conjugates. Biochemistry 18:690–697
Mann S, Banninster JV, Williams RJP (1986) Structure and composition of ferritin cores isolated from human spleen, limpet (Patella vulgata) haemolymph and bacterial (Pseudomonas aeruginosa) cells. J Mol Biol 188:225–232
Mann S, Williams JM, Treffry A, Harrison PM (1987) Reconstituted and native iron-cores of bacterioferritin and ferritin. J Mol Biol 198:405–416a
McKay RH, Fineberg RA (1964a) Horse spleen haemosiderin, I. Isolation. Arch Biochem Biophys 104:487–495
McKay RH, Fineberg RA (1964b) Horse spleen haemosiderin, II. Further characterization. Arch Biochem Biophys 104:496–508
O'Connell MJ, Ward RJ, Baum H, Peters TJ (1985) The role of iron in ferritin- and haemosiderin-mediated lipid peroxidation in lysosomes. Biochem J 229:135–139
O'Connell MJ, Halliwell B, Moorhouse CP, Aruoma 0I, Baum H, Peters TJ (1986) Formation of hydroxyl radicals in the presence of ferritin and haemosiderin. Biochem J 234:727–731
Richter GW (1959) The cellular transformation of injected colloidal iron complexes into ferritin and haemosiderin in experimental animals. A study with the aid of electron microscopy. J Exp Med 109:197–216
Richter GW (1984) Studies of iron overload. Rat liver siderosome ferritin. Lab Invest 50:26–35
Rimbert JN, Dumas F, Kellersohn C, Girot R, Brissot P (1985) Mössbauer spectroscopy of iron overloaded livers. Biochimie 67:663–668
St. Pierre TG, Bell SH, Dickson DPE, Mann S, Webb J, Moore RG, Williams P (1986) Mössbauer spectroscopic studies of the cores of human, limpet and bacterial ferritins. Biochim Biophys Acta 870:127–134
Theil EC (1987) Ferritin: structure, gene regulation and cellular functions in animals, plants and microorganisms. Annu Rev Biochem 56:289–315
Towe KM (1981) Structural distinction between ferritin and iron dextran (imferon). J Biol Chem 256:9377–9378
Treffry A, Lee PJ, Harrison PM (1984) Iron-induced changes in rat liver isoferritins. Biochem J 220:717–722
Treffry A, Harrison PM, Cleton MI, De Bruijn WC, Mann S (1987) A note on the composition and properties of ferritin iron-cores. J Inorg Biochem 31:1–6
Walter G, Scheidtmann K-H, Carbone A, Laudano AP, Doolittle RF (1980) Antibodies specific for the carboxy- and amino-terminal regions of simian virus 40 large tumor antigen. Proc Nail Acad Sci USA 77:5197–5200
Ward RJ, O'Connell MJ, Mann S, Wade VJ, Reid NMK, Dickson DPE, Bomford A, Peters TJ (1988) Heterogeneity of iron cores in hepatic haemosiderins from primary and secondary haemochromatosis. Biochem Soc Trans (in press)
Weir MP, Gibson JF, Peters TJ (1984) Biochemical studies on the isolation and characterization of human spleem haemosiderin. Biochem J 223:31–38
Wixom RL, Prutkin L, Munro HN (1980) Haemosiderin: nature, formation and significance. Int Rev Exp Pathol 22:193–224
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Andrews, S.C., Brady, M.C., Treffry, A. et al. Studies on haemosiderin and ferritin from iron-loaded rat liver. Biol Metals 1, 33–42 (1988). https://doi.org/10.1007/BF01128015
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01128015