Summary
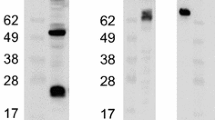
Rabbit haemorrhagic disease virus (RHDV) capsid protein was expressed in a baculovirus system. Analysis of the expressed product showed that the recombinant protein, which is 60 kDa in size, was antigenic as revealed by its reactions in ELISA and Western blot with the antibodies raised against RHDV. Direct electron microscopy of the cell culture supernatant and the purified protein demonstrated that the capsid protein expressed in insect cells self-assembled to form empty virus-like particles (VLP) which are similar in size and morphology to that of native virus. These particles were immunoreactive with polyclonal anti-RHDV antibodies and with four monoclonal antibodies which recognise conformational epitopes of the virus. The results indicated that the VLPs were morphologically and antigenically indistinguishable from native virus. The recombinant VLPs induced high levels of RHDV-specific antibodies in rabbits and mice following immunisation. The immune response to the VLPs protected the rabbits following challenge with the virulent RHDV. In haemagglutination assays, the VLPs bound to human red blood cells similar to the native virus particles. The recombinant protein and or VLPs is suitable for the development of a rapid, sensitive and reliable test for detection of antibodies to RHDV and for use as a vaccine for domestic rabbits.
Similar content being viewed by others
References
Arguello Villares JL (1991) Viral haemorrhagic disease of rabbits: vaccination and immune response. Rev Sci Tech 10: 471–480
Beeh P (1992) The deadly seven running riot over native species. Geo 14: 48–65
Bell A (1987) New strategies in the rabbit war. Ecos 53: 12–18
Boga JA, Casais R, Marin MS, Martin-Alonso JM, Carmenes RS, Prieto M, Parra F (1994) Molecular cloning, sequencing and expression inEscherichia coli of the capsid protein gene from rabbit haemorrhagic diseas virus (Spanish isolate AST/89). J Gen Virol 75: 2409–2413
Boga JA, Marin MS, Casais R, Prieto M, Parra F (1992) In vitro translation of a subgenomic mRNA from purified virions of the Spanish field isolate AST/89 of rabbit hemorrhagic disease virus (RHDV). Virus Res 26: 33–40
Brown CS, Van Lent JWM, Vlak JM, Spaan WJM (1991) Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J Virol 65: 2702–2706
Carter MJ, Routledge EG, Toms GL (1989) Monoclonal antibodies to feline calicivirus. J Gen Virol 70: 197–2200
Creagh C (1992a) New approaches to rabbit and fox control. Ecos 71: 18–24
Creagh C (1992b) Rehabilitating degraded rangelands. Ecos 73: 27–31
Estes MK, Crawford SE, Penaranda ME, Petrie BL, Burns JW, Chan W-K, Ericson B, Smith GE, Summers MD (1987) Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol 61: 1488–1494
Greenberg HB, Valdesuso JR, Kalica AR, Wyatt RG, McAuliffe VJ, Kapikian AZ, Chanock RM (1981) Proteins of Norwalk virus. J Virol 37: 994–999
Jiang X, Wang M, Graham DY, Estes MK (1992) Expression, self-assembly, and antigenicity of Norwalk virus capsid protein. J Virol 66: 6527–6532
Jiang X, Wang M, Wang K, Estes MK (1993) Sequence and genomic organization of Norwalk virus. Virology 195: 51–61
King LA, Possee RD (1992) The baculovirus expression system: a laboratory guide. Chapman and Hall, London
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Laurent S, Vautherot J-F, Madelaine M-F, Le Gall G, Rasschaert D (1994) Recombinant rabbit hemorrhagic disease virus capsid protein expressed in baculovirus self-assembles into virus-like particles and induces protection. J Virol 68: 6794–6798
Lenghaus C, Studdert MJ (1980) Relationships of canine panleucopaenia(enteritis) and myocarditis parvoviruses to feline panleucopaenia virus. Aust Vet J 56: 152–153
Liu SJ, Xue HP, Pu BQ, Quian NH (1984) A new viral disease in rabbits. Anim Husb Vet Med 16: 253–255
Luckow VA, Summers MD (1989) High level expression of nonfused foreign genes withAutographa californica nuclear poyhedrosis virus expression vectors. Virology 170: 31–39
Lunt RA, White JR, Blacksell SD (1988) Evaluation of a monoclonal antibody blocking ELISA for the detection of group-specific antibodies to bluetongue virus in experimental and field sera. J Gen Virol 69: 2729–2740
Meyers G, Wirblich C, Thiel H-J (1991a) Rabbit hemorrhagic disease virus-molecular cloning and nucleotide sequencing of a calicivirus genome. Virology 184: 664–676
Meyers G, Wirblich C, Thiel H-J (1991b) Genomic and subgenomic RNAs of rabbit hemorrhagic disease virus are both protein-linked and packaged into particles. Virology 184: 677–686
Milton ID, Vlasak R, Nowotny N, Rodak L, Carter MJ (1992) Genomic 3′ terminal sequence comparison of three isolates of rabbit haemorrhagic disease virus. FEMS Microbiol Lett 93: 37–42
Morisse JP, Le Gall G, Boilletot E (1991) Hepatitis of viral origin inLeporidae: introduction and aetiological hypotheses. Rev Sci Tech 10: 283–295
Neill JD (1990) Nucleotide sequence of a region of the feline calicivirus genome which encodes picornavirus-like RNA dependent RNA polymerase, cysteine protease and 2C polypeptides. Virus Res 17: 145–160
Neill JD (1992) Nucleotide sequence of the capsid protein gene of two serotypes of San Miguel sea lion virus: identification of conserved and non-conserved amino acid sequences among calicivirus capsid proteins. Virus Res 24: 211–222
Ohlinger VF, Haas B, Meyers G, Weiland F, Thiel H-J (1990) Identification and characterization of the virus causing rabbit hemorrhagic disease. J Virol 64: 3331–3336
Parra F, Boga JA, Marin MS, Casais R (1993) The amino terminal sequence of VP60 from rabbit hemorrhagic disease virus supports its putative subgenomic origin. Virus Res 27: 219–228
Parra F, Prieto M (1990) Purification and characterization of a calicivirus as the causative agent of a lethal hemorrhagic disease in rabbits. J Virol 64: 4013–4015
Prasad BVV, Rothnagel R, Jiang X, Estes MK (1994) Three dimensional structure of baculovirus-expressed Norwalk virus capsids. J Virol 68: 5117–5125
Rodak L, Granatova M, Valicek L, Smid B, Vesely T, Nevorankova Z (1990) Monoclonal antibodies to rabbit haemorrhagic disease virus and their use in the diagnosis of infection. J Gen Virol 71: 2593–2598
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487–491
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Schaffer FL, Bachrach HL, Brown F, Gillespie JH, Burroughs JN, Madin SH, Madeley CR, Povey RC, Scott F, Smith AW, Studdert MJ (1980)Caliciviridae. Intervirology 14: 1–6
Schaffer FL, Soergel ME (1976) Single major polypeptide of a calicivirus: characterization by polyacrylamide gel electrophoresis and stabilization of virions by cross linking with dimethyl suberimidate. J Virol 19: 925–931
Studdert MJ (1978) Caliciviruses: brief review. Arch Virol 58: 157–191
Studdert MJ (1994) Rabbit haemorrhagic disease virus: a calicivirus with differences. Aust Vet J 71: 264–266
Urakawa T, Ferguson M, Minor PD, Cooper J, Sullivan M, Almond JW, Bishop DHL (1989) Synthesis of immunogenic, but non-infectious, poliovirus particles in insect cells by a baculovirus expression vector. J Gen Virol 70: 1453–1463
Valicek L, Smid B, Rodak L, Kudrna J (1990) Electron and immunoelectron microscopy of rabbit haemorrhagic disease virus (RHDV). Arch Virol 112: 271–275
Webb NR, Summers MD (1990) Expression of proteins using recombinant baculovirus. Technique 2: 173–188
Westbury HA (1989) Viral haemorrhagic disease of rabbits. In: report of study tour, Committee of Nature Conservation Ministers of Australia and New Zealand, Geelong, Victoria, Australia
Wang LF, Du Plessis DH, White JR, Hyatt AD, Eaton BT (1995) Use of a genetargeted phage display random library to map an antigenic determinant on the bluetongue virus outer capsid protein VP5. J Immunol Methods 178: 1–12
Xu ZJ, Chen WX (1989) Viral haemorrhagic disease in rabbits: Vet Res Commun 13: 205–212
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nagesha, H.S., Wang, L.F., Hyatt, A.D. et al. Self-assembly, antigenicity, and immunogenicity of the rabbit haemorrhagic disease virus (Czechoslovakian strain V-351) capsid protein expressed in baculovirus. Archives of Virology 140, 1095–1108 (1995). https://doi.org/10.1007/BF01315418
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01315418