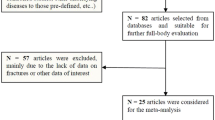
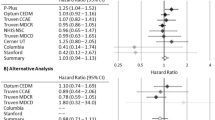
Abstract
The Fracture Intervention Trial (FIT) is a randomized, double-masked, placebo-controlled trial designed to test the hypothesis that alendronate, an amino-bisphosphonate, will reduce the rate of fractures in women aged 55–80 years with low hip bone marrow density (<0.68 gm/cm2 at the femoral neck). It is being conducted at 11 clinical centers around the United States with a coordinating center at UC San Francisco. The goal was to randomize 6000 women. When recruitment was completed (in May 1993), 6457 women had been randomized, amounting to 108% of goal. The women were assigned to one of two substudies. The first (Vertebral Deformity study) includes 2023 women who have at least one vertebral deformity, and will test the hypothesis that alendronate reduces the rate of new vertebral deformities during 3 years of follow-up. This substudy has a power of 0.90 to detect a 32% reduction in the incidence of new vertebral deformities, assuming a 6.5% annual incidence of new vertebral deformities in the placebo group. The second study (Clinical Fracture study) includes 4434 women without vertebral deformities at baseline and will test the hypothesis that alendronate reduces the rate of clinically recognized fractures of all types over an average of 4.25 years of follow-up. This substudy has a 0.90 power to detect a 25% reduction in the rate of all clinical fractures, assuming 4% annual incidence in the placebo group. To our knowledge, this is the largest prospective, randomized, controlled study undertaken to determine the effectiveness of a treatment in reducing the risk of fractures in postmenopausal women.
Similar content being viewed by others
References
Ross P, Davis J, Vagel J, Wasnich R. A critical review of bone mass and the risk of fractures in osteoporosis. Calcif Tissue Int 1990;46:149–61.
Black D, Cummings S, Melton L III. Appendicular bone mineral and a woman's lifetime risk of hip fracture. J Bone Miner Res 1992;7:639–46.
Cummings S, Black D, Nevitt M, Browner W, Cauley J, Ensrud K, et al. Bone density at various sites for prediction of hip fractures. Lancet 1993;341:72–5.
Riggs BL, Hodgson SF, O'Fallon WM, Chao EYS, Wahner HW, Muhs JM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990;322:802–9.
Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PG, Jackson RD, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med 1990;323:73–9.
Ettinger B, Genant HK, Cann CE. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med 1985;102:319–24.
Kiel DP, Felson DT, Anderson JJ, Wilson PWF, Moskowitz MA. Hip fracture and the use of estrogens in postmenopausal women. N Engl J Med, 1987;317:1169–74.
Grady D, Rubin S, Petitti D, Fox C, Black D, Ettinger B, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 1992;117:1016–37.
Chapuy M, Arlot M, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992;327:1637–42.
Overgaard K, Hansen MA, Jensen SB, Christiansen C. Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ 1982;305:556–61.
Storm T, Thamsborg G, Steiniche T, Genant HK, Sorenson OH. Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med 1990;322:1265–71.
Cummings SR, Block G, McHenry K, and Baron RB. Evaluation of two food frequency methods of measuring dietary calcium intake. Am J Epidemiol 1987;126:796–802.
Riggs BL and Melton LJ III. Involutional osteoporosis. N Engl J Med 1986;314:1676–84.
Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, et al. Appendicular bone density and age predict hip fracture in women. JAMA 1990;263:665–8.
Seeley D, Browner W, Nevitt M, Genant H, Scott J, Cummings S. Which fractures are associated with low appendicular bone mass in elderly women? Ann Intern Med 1991;115:837–42.
Ettinger B, Block JE, Smith R, Cummings SR, Harris ST, Genant HK. An examination of the association between vertebral deformities, physical disabilities and psycho-social problems. Maturitas 1988;8:283–96.
Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991;114:919–23.
Black D, Cummings S, Genant H, Nevitt M, Palermo L, Browner W. Axial and appendicular bone density predict fractures in older women. J Bone Miner Res 1992;7:633–8.
Mazess RB, Barden H, Ettinger M, Schultz E, Bone density of the radius, spine, and proximal femur in osteoporosis. J Bone Miner Res 1988;3:13–8.
Melton LJ, Kan SH, Frye MA, Wahner HW, O'Fallon WM, Riggs BL. Epidemiology of vertebral fractures in women. Am J Epidemiol 1989;129:1000–11.
Black DM, Cummings SR, Stone K, Hudes E, Palermo L, Steiger P. A new approach to defining normal vertebral dimensions. J Bone Miner Res 1991;8:883–92.
Friedman LM, Furberg CD, DeMets DL, Fundamentals of clinical trials. 2nd ed. St. Louis, MO: Mosby Year Book, 1985:302.
Lan KKG and DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–63.
Casagrande JT, Pike MC, Smith PG. The power function of the ‘exact’ test for comparing two binomial proportions. Appl Statistics 1978;27:176–81.
Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, Loss to follow-up, noncompliance and stratification. Biometrics 1986;42:507–19.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Black, D.M., Reiss, T.R., Nevitt, M.C. et al. Design of the Fracture Intervention Trial. Osteoporosis Int 3 (Suppl 3), 29–39 (1993). https://doi.org/10.1007/BF01623005
Issue Date:
DOI: https://doi.org/10.1007/BF01623005