Summary
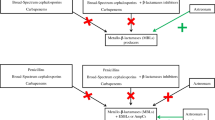
A plasmid-encoded β-lactamase conferring extended broad spectrum resistance including cephamycins was identified in aKlebsiella pneumoniae strain isolated from a patient's wound. Strains harbouring the plasmid pMVP-1 were resistant to penicillins, cephalosporins of all generations (parenteral and new oral compounds) cephamycins, aztreonam, tetracycline, chloramphenicol, sulfonamides and to all aminoglycosides modified by AAC-(6)-I-transferase. β-lactams still active against these strains were temocillin, ceftazidime, cefpirome, carumonam and the carbapenems imipenem and meropenem. The new cephamycinase (CMY-1) was more strongly inhibited by sulbactam in the majority of combinations than by clavulanic acid or tazobactam. MICs of ceftazidime and carumonam were not reduced by inhibitors in the wild type and the transconjugant. A transferable plasmid (pMVP-1) of about 9.6 × 107 dalton was demonstrated by gel-electrophoresis. In the wild type and the transconjugant a β-lactamase with an isoelectric point of 8.0 was identified. This enzyme CMY-1 is different from the other extended broad spectrum β-lactamases (TEM-3 to TEM-10, SHV-2 to SHV-5). The incidence of this enzyme may be underestimated, since resistance to cephamycins in Klebsiella andEscherichia coli has so far been regarded as almost exclusively chromosomally encoded and sensitivity of CMY-1 to clavulanic acid is low. Therefore, screening for CMY-1 beta-lactamases by the usual double disk test including clavulanic acid is not sensitive enough to detect CMY-1 producers. Sulbactam (e. g. in combination with ampicillin) disks and a cephamycin should therefore be used as well when screening for super extended broad spectrum (SEBS-) beta-lactamases.
Zusammenfassung
Eine plasmidcodierte Betalaktamase, deren Betalaktamresistenz im Gegensatz zu den bisherigen Betalaktamasen mit erweitertem Breitspektrum zusätzlich auch zu Resistenz gegenüber Cephamycinen führt, wurde in einem Wundisolat vonKlebsiella pneumoniae identifiziert. Der WildtypstammK. pneumoniae CHO sowie derEscherichia coli Transkonjugant waren resistent gegenüber Penicillinen, Cephalosporinen aller Generationen (parenterale und neue orale) und Aztreonam. Noch wirksam blieben Temocillin, Ceftazidim, Cefpirom und Carumonam, sowie die Carbapeneme Imipenem und Meropenem. Unter den Betalaktamaseinhibitoren war Sulbactam in der Mehrzahl der Kombinationen besser wirksam als Clavulansäure oder Tazobactam. Die minimale Hemmkonzentration von Ceftazidim und Carumonam blieben in Gegenwart von Betalaktamaseinhibitoren unverändert, trotz extrachromosomaler Lokalisation des Resistenzgens. Ein transferierbares Plasmid (pMVP) mit etwa 9.6 × 107 Dalton wurde nachgewiesen. In Wildtyp und Transkonjuganten konnte eine Betalaktamase mit einem isoelektrischen Punkt von 8.0 identifiziert werden. Diese Cephamycinase (CMY-1) unterscheidet sich von den übrigen Betalaktamasen mit erweitertem Breitspektrum. (TEM-2 bis TEM-10, SHV-2 bis SHV-5). Die Verbreitung von plasmidcodierten Cephamycinasen wird möglicherweise unterschätzt, da Resistenz gegenüber Cephamycinen bisher als chromosomal kodiert erachtet wird und in Suchtesten nach Betalaktamasen mit erweitertem Breitspektrum Clavulansäure mit nur schwacher Wirkung gegenüber der Cephamycinase Verwendung finden.
Similar content being viewed by others
References
Philippon, A., Labia, R., Jacoby, G. Extended spectrum β-lactamases. Antimicrob. Agents Chemother. 33 (1989) 1131–1136.
Jarlier, V., Nicolas, M.-H., Fournier, G., Philippon, A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10 (1988) 867–878.
Bauernfeind, A., Shah, P., Petermüller, C., Motz, M.: Plasmid-determined resistance to third generation cephalosporins in Enterobacteria. IV Mediterranean Congress of Chemotherapy (1984). Proceedings 30–31.
Shah, P. M., Stille, W. Escherichia coli andKlebsiella pneumoniae strains more susceptible to cefoxitin than to third generation cephalosporins. J. Antimicrob. Chemother. 11 (1983) 597–601.
Buré, A., Legrand, P., Arlet, G., Jarlier, V., Paul, G., Philippon, A. Dissemination in five French hospitals ofKlebsiella pneumoniae serotype K25 harbouring a new transferable enzymatic resistance to third generation cephalosporins and aztreonam. Eur. J. Clin. Microbiol. Infect. Dis. 7 (1988) 780–782.
Gutmann, L., Ferré, B., Goldstein, F. W., Rizk, N., Pinto-Schuster, E., Acar, J. F., Collatz, E. SHV-5, a novel SHV-type β-lactamase that hydrolyzes broad spectrum cephalosporins and monobactams. Antimicrob. Agents Chemother. 33 (1989) 951–956.
Rubin, L. G., Medeiros, A. A., Yolken, R. H., Moxon, E. R. Ampicillin treatment failure of apparently β-lactamase-negativeHaemophilus influenzae type b meningitis due to novel β-lactamase. Lancet ii (1981) 1008–1010.
Matthew, M., Harris, A. M., Marshall, M. J., Ross, G. W. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88 (1975) 169–178.
O'Callaghan, C. H., Morris, A., Kirby, S. M., Shingler, A. H. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1 (1972) 283–288.
Kado, C. I., Liu, S.-T. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145 (1981) 1365–1373.
Meyers, J. A., Sanchez, D., Elwell, L. P., Falkow, S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J. Bacteriol. 127 (1976) 1529–1537.
Thabaut, A., Acar, J., Arlet, G., Berardi-Grassias, L., Bergogne-Bérézin, E., Brun, Y., Buisson, Y., Chabanon, G., Cluzel, R., Courtier, A., Dabernat, H., Duval, J., Fleurette, J., Ghnassia, J. C., Jarlier, V., Meyran, M., Monteil, H., Petithory, J. C., Philippon, A., Sedallian, A., Sirot, J., Viot, M., Werneburg, B. Sensibilité desPseudomonas aeruginosa et des Klebsiella à la ceftazidime. Presse Médicale 37 (1988) 1895–1899.
Casellas, J. M., Goldberg, M.: Incidence of strains producing extended spectrum beta-lactamases in Argentina. Infection, in print.
Bauernfeind, A., Hörl, G. Novel R-factor borne β-lactamase ofEscherichia coli confering resistance to cephalosporins. Infection 15 (1987), 257–259.
Knothe, H., Shah, P., Krčemery, V., Antal, M., Mitsuhashi, S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates ofKlebsiella pneumoniae andSerratia marcescens. Infection 11 (1983) 35–37.
Philippon, A., Paul, G., Vedel, G., Nevot, P. Résistance plasmidique aux céphalosporines de 3è génération. Presse Médicale, 37 (1988) 1883–1889.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bauernfeind, A., Schweighart, S. & Chong, Y. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection 17, 316–321 (1989). https://doi.org/10.1007/BF01650718
Issue Date:
DOI: https://doi.org/10.1007/BF01650718