Abstract
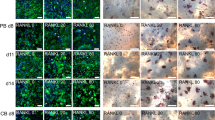
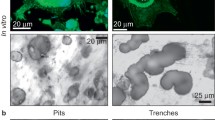
Several possible roles have been proposed for osteocytes in bone metabolism, but the precise functions of these cells are still obscure. In this study, we examined the effects of osteocytes on osteoclast formation, using isolated osteocytes from 16-day-old chick embryonic parietal bones and mouse hemopoietic blast cells from spleen cells obtained from 5-fluorouracil-treated mice. Purity of the isolated osteocytes was more than 95%, and their osteocytic phenotypes—such as those having a typical stellate shape and being immunoreactive for osteocyte-specific monoclonal antibody OB 7.3, the nonproliferative phenotype, and those negative for alkaline phosphatase (ALP) activity—were retained during 3 days of culture. When hemopoietic blast cells were cocultured with the isolated osteocytes, the number of osteoclastic tartrate-resistant acid phosphatase (TRAP)-positive multinucleate cells (MNCs) from the blast cells increased even in the absence of any osteotropic factors. This stimulatory effect depended on the number of osteocytes added, and was consistent with that obtained with bone fragments freed of nonosteocytic cells by stripping the periosteum. Conditioned medium (CM) of isolated osteocytes also had stimulatory effects on both formation and bone-resorbing activity of the MNCs, indicating that osteocytes increased osteoclastic development, at least in part, via their production of some soluble factor(s). This osteoclast-inducing activity in the osteocyte CM was observed in the fraction that eluted at around 0.3 M NaCl from a mono-Q anion-exchange column, and this elution profile was similar to that of cell lysates of freshly isolated osteocytes and of stripped bone fragments, indicating the existence of such osteoclastogenesis factor(s) in vivo. Finally, both the osteocyte CM and the active fraction of mono-Q chromatography increased the pit area on dentine slices in the cultures of unfractionated mouse bone cells as well. Thus, osteocytes may play a role in the physiological processes of osteoclastic bone resorption.
Similar content being viewed by others
References
Palumbo C (1986) A three-dimensional ultrastructural study of osteoidosteocytes in the tibia of chick embryos. Cell Tissue Res 246:125–131
Marotti G, Ferretti M, Muglia MA, Palumbo C, Palazzini S (1992) A quantitative evaluation of osteoblast-osteocyte relationships on growing endosteal surface of rabbit tibiae. Bone 13:363–368
Elmardi AS, Katchburian MV, Katchburian E (1990) Electron microscopy of developing calvaria reveals images that suggest that osteoclasts engulf and destroy osteocytes during bone resorption. Calcif Tissue Int 46:239–245
Bloom W, Fawcett DW (1975) Bone. In: A textbook of histology, 10th edn. Saunders, Philadelphia, pp 244–287
Belanger LF (1969) Osteocytic osteolysis. Calcif Tissue Res 4:1–12
Palumbo C, Palazzini S, Marotti G (1990) Morphological study of intercellular junctions during osteocyte differentiation. Bone 11:401–406
Palumbo C, Palazzini S, Zaffe D, Marotti G (1990) Osteocyte differentiation in the tibia of newborn rabbit: An ultra-structural study of the formation of cytoplasmic processes. Acta Anat 137:350–358
Chambers TJ (1989) The origin of the osteoclast. In: Peck WA (ed) Bone and mineral research, annual 6. Elsevier, Amsterdam, pp 1–25
Mundy GR (1993) Local control of osteoclast function. In: Lindsay R, Meunier PJ (eds) Osteoporosis international. Proceedings of the international conference on osteoporsis. Dorset, Dorchester, UK, 3 [Suppl 1]:S126–127
Mundy GR (1993) Bone resorbing cells. In: Favus MJ (ed) Primer on the metabolic bone diseases and disorders of mineral metabolisms, 2nd edn. Raven, New York, pp 25–32
Kurihara N, Suda T, Miura Y, Nakauchi H, KodamaH, Hiura K, Hakeda Y, Kumegawa M (1989) Generation of osteoclasts from isolated hematopoietic progenitor cells. Blood 74:1295–1302
Kerby JA, Hattersley G, Collins DA, Chambers TJ (1992) Derivation of osteoclasts from hematopoietic colony-forming cells in culture. J Bone Miner Res 7:353–362
Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T (1989) The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology 125:1805–1813
Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T (1990) Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA 87:7260–7264
Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T (1988) Osteoblastic cells are involved in osteoclast formation. Endocrinology 123:2600–2602
Schneider GB, Relfson M (1988) A bone marrow fraction enriched for granulocyte-macrophage progenitors gives rise to osteoclasts in vitro. Bone 9:303–308
Braidman IP, Anderson DC (1993) Role of bone matrix in osteoclast recruitment in cultured fetal rat calvariae. J Bone Miner Res 8:231–238
Burger EH, Van Der Meer JWM, Nijiweide PJ (1984) Osteoclast formation from mononuclear phagocytes: role of bone-forming cells. J Cell Biol 99:1901–1906
Nijweide PJ, Mulder RJP (1986) Identification of osteocytes in osteoblast-like cell cultures using a monoclonal antibody specifically directed against osteocytes. Histochemistry 84:342–347
Takada Y, Kusuda M, Hiura K, Sato T, Mochizuki H, Nagao Y, Tomura M, Yahiro M, Hakeda Y, Kawashima H, Kumegawa M (1992) A simple method to assess osteoclast-mediated bone resorption using unfractionated bone cells. Bone Min 17:347–359
Kodama H, Sudo H, Koyama H, Kasai S, Yamamoto S (1984) In vitro hemopoiesis within a microenvironment created by MC3T3-G2/PA6 preadipocytes. J Cell Physiol 118:233–240
Sato T (1993) Prostaglandin E2 indirectly stimulates via stromal cells, but directly inhibits, osteoclastic cell formation (in Japanese). J Meikai Univ Sch Dent 22:56–66
Oursler MJ, Osdoby P (1988) Osteoclast develpment in marrow cultured in calvaria-conditioned media. Dev Biol 127:170–178
Hiura K, Sumitani K, Kawata T, Higashino K, Okawa M, Sato T, Hakeda Y, Kumegawa M (1991) Mouse osteoblastic cells (MC3T3-E1) at different stages of differentiation have apposite effects on osteoclastic cell formation. Endocrinology 128: 1630–1637
Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatus T, Stanley ER, Kurokawa T, Suda T (1993) Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest 91:257–263
Manolagas SC, Jilka RL, Girasole G, Passeri G, Bellido T (1993) Estrogen, cytokines, and the control of osteoclast formation and bone resorption in vitro and in vivo. In: Lindsay R, Meunier PJ (eds) Osteoporosis international. Proceedings of the international conference on osteoporosis. Dorset, Dorchester, UK, 3 [Suppl 1]:S114–116
Felix R, Cecchini MG, Hofstetter W, Guenther HL, Fleisch H: (1991) Production of granulocyte-macrophage (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) by rat clonal osteoblastic cell population CRP 10/30 and the immortalized cell line IRC 10/30 mycl stimulated by tumor necrosis factor α. Endocrinology 126:661–667
Elford PR, Felix R, Cecchini M, Trechsel U, Fleisch H (1987) Murine osteoblastlike cells and the osteogenic cell MC3T3-E1 release a macrophage colony-stimulating activity in culture. Calcif Tissue Int 41:151–156
Keeting PE, Rifas L. Harris SA, Colvard DS, Spelsberg TC, Peck WA, Riggs BL (1991) Evidence for interleukin-1β production by cultured normal human osteoblast-like cells. J Bone Miner Res 6:827–833
Littlewood AJ, Russell J, Harvey GR, Hughes DE, Russell RGG, Gowen M: (1991) The modulation of the expression of IL-6 and its receptor in human osteoblasts in vitro. Endocrinology 129:1513–1520
Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC (1992) Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science 257:88–91
Author information
Authors and Affiliations
About this article
Cite this article
Tanaka, K., Yamaguchi, Y. & Hakeda, Y. Isolated chick osteocytes stimulate formation and bone-resorbing activity of osteoclast-like cells. J Bone Miner Metab 13, 61–70 (1995). https://doi.org/10.1007/BF01771319
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01771319