Abstract
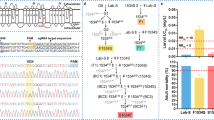
We report the isolation of cDNA clones containing the full 6.3-kb coding sequence of thepara-type sodium channel gene of the housefly,Musca domestica. This gene has been implicated as the site of knockdown resistance (kdr), an important resistance mechanism that confers nerve insensitivity to DDT and pyrethroid insecticides. The cDNAs predict a polypeptide of 2108 amino acids with close sequence homology (92% identity) to theDrosophila para sodium channel, and around 50% homology to vertebrate sodium channels. Only one major splice form of the housefly sodium channel was detected, in contrast to theDrosophila para transcript which has been reported to undergo extensive alternative splicing. Comparative sequence analysis of housefly strains carryingkdr or the more potentsuper-kdr factor revealed two amino acid mutations that correlate with these resistance phenotypes. Both mutations are located in domain II of the sodium channel. A leucine to phenylalanine replacement in the hydrophobic IIS6 transmembrane segment was found in two independentkdr strains and sixsuper-kdr strains of diverse geographic origin, while an additional methionine to threonine replacement within the intracellular IIS4-S5 loop was found only in thesuper-kdr strains. Neither mutation was present in five pyrethroid-sensitive strains. The mutations suggest a binding site for pyrethroids at the intracellular mouth of the channel pore in a region known to be important for channel inactivation.
Similar content being viewed by others
References
Amichot M, Castella C, Cuany A, Berge JB, Pauron D (1992) Target modification as a molecular mechanism of pyrethroid resistance inDrosophila melanogaster. Pesticide Biochem Physiol 44:183–190
Bell CA, Williamson MS, Denholm I, Devonshire AL (1995) Restriction fragment length polymorphism analysis of a sodium channel gene locus in susceptible and knockdown resistant houseflies,Musca domestica. In: Clark JM (ed) Molecular action of insecticides on ion channels, ACS Symposium Series 591. American Chemical Society, Washington DC, pp 86–96
Bertioli DJ, Burrows PR (1995) A simple RACE method based on CTAB precipitation. Methods Mol Cell Biol 5:118–121
Bloomquist JR (1993) Neuroreceptor mechanisms in pyrethroid mode of action and resistance. In: Roe M, Kuhr RJ (eds) Reviews in pesticide toxicology. Toxicology Communications, Raleigh NC, pp 181–226
Bloomquist JR, Miller TA (1986) Sodium channel neurotoxins as probes of the knockdown resistance mechanism. Neuro Toxicology 7:217–224
Bloomquist JR, Soderlund DM, Knipple DC (1989) Knockdown resistance to DDT and pyrethroid insecticides in thenap ts mutant ofDrosophila melanogaster is correlated with reduced neuronal sensitivity. Arch Insect Biochem Physiol 10:293–302
Brown AM (1993) Functional bases for interpreting amino acid sequences of voltage-dependent K+ channels. Annu Rev Biophys Biomol Struct 22:173–198
Busvine JR (1951) Mechanism of resistance to insecticide in houseflies. Nature 168:193–195
Catterall WA (1988) Structure and function of voltage-sensitive ion channels. Science 242:50–61
Catterall WA (1993) Structure and function of voltage-gated ion channels. Trends Neurosci 16:500–506
Choi KL, Mossman C, Aube J, Yellen G (1993) The internal quaternary ammonium receptor site ofShaker potassium channels. Neuron 10:533–541
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Dong K, Scott JG (1994) Linkage ofkdr-type resistance and thepara homologous sodium channel gene in German cockroaches (Blattella germanica). Insect Biochem Mol Biol 24:647–654
Elliott M, Janes NF, Potter C (1978) The future of pyrethroids in insect control. Annu Rev Entomol 23:443–469
Farnham AW (1977) Genetics of resistance of houseflies (Musca domestica) to pyrethroids. I. Knockdown resistance. Pesticide Sci 8:631–636
Farnham AW, Khambay BPS (1995) The pyrethrins and related compounds. 39 Structure-activity relationships of pyrethroidal esters with cyclic side-chains in the alcohol component against resistant strains of housefly (Musca domestica). Pesticide Sci 44:269–275
Farnham AW, Murray AWA, Sawicki RM, Denholm I, White JC (1987) Characterization of the structure-activity relationship ofkdr and two variants of super-kdr to pyrethroids in the housefly (Musca domestica). Pesticide Sci 19:209–220
ffrench-Constant RH (1994) The molecular and population genetics of cyclodiene insecticide resistance. Insect Biochem Mol Biol 24:335–345
ffrench-Constant RH, Rocheleau TA, Steichen JC, Chalmers AE (1993) A point mutation in aDrosophila GABA receptor confers insecticide resistance. Nature 363:449–451
Georghiou GP (1990) Overview of insecticide resistance. In: Green MB, LeBaron HM, Moberg WK (eds) Managing resistance to agrochemicals. American Chemical Society, Washington DC, pp 18–41
Grubs RE, Adams PM, Soderlund DM (1988) Binding of [3H] saxitoxin to head membrane preparations from susceptible and knockdown resistant houseflies. Pesticide Biochem Physiol 32:217–223
Hong CS, Ganetzky B (1994) Spatial and temporal expression patterns of two sodium channel genes inDrosophila. J Neurosci 14:5160–5169
Hoyer RF, Plapp FW (1966) A gross genetic analysis of two DDT resistant housefly strains. J Econ Entomol 59:495–501
Isacoff EY, Jan YN, Jan LY (1991) Putative receptor for the cytoplasmic inactivation gate in theShaker K+ channel. Nature 353:86–90
Kallen RG, Cohen SA, Barchi RL (1993) Structure, function and expression of voltage dependent sodium channels. Mol Neurobiol 7:383–428
Kasbekar DP, Hall LM (1988) ADrosophila mutation that reduces sodium channel number confers resistance to pyrethroid insecticides. Pesticide Biochem Physiol 32:135–145
Knipple DC, Doyle KE, Marsella Herrick PA, Soderlund DM (1994) Tight genetic linkage between thekdr insecticide resistance trait and a voltage-sensitive sodium channel gene in the house fly. Proc Natl Acad Sci USA 91:2483–2487
Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Analytical Biochem 163:16–20
Lopez GA, Jan YN, Jan LY (1994) Evidence that the S6 segment of theShaker voltage-gated K+ channel comprises part of the pore. Nature 367:179–182
Loughney K, Kreber R, Ganetzky B (1989) Molecular analysis of thepara locus, a sodium channel gene inDrosophila. Cell 58:1143–1154
Lund AE, Narahashi T (1983) Kinetics of sodium channel modification as the basis for the variation in the nerve membrane effects of pyrethroids and DDT analogues. Pesticide Biochem Physiol 20:203–216
McClatchey AI, McKennayasek D, Cros D, Worthen HG, Kuncl RW, DeSilva SM, Cornblath DR, Gusella JF, Brown RH (1992) Novel mutations in families with unusual and variable disorders of the skeletal muscle sodium channel. Nature Genet 2:148–152
McPhee JC, Ragsdale DS, Scheuer T, Catterall WA (1994) A mutation in segment IVS6 disrupts fast inactivation of sodium channels. Proc Natl Acad Sci USA 91:12346–12350
Milani R (1954) Comportamento mendeliano della resistenza alla azione abbattante del DDT: correlazione tran abbattimento e mortalia in Musca domestica L Riv Parasitol 15:513–542
Mullaley A, Taylor R (1994) Conformational properties of pyrethroids. J Computer-Aided Design 8:135–152
Mutero A, Pralavorio M, Bride JM, Fournier D (1994) Resistance associated point mutations in insecticide-insensitive acetyl-cholinesterase. Proc Natl Acad Sci USA 91:5922–5926
Narahashi T (1992) Nerve membrane Na+ channels as targets of insecticides. Trends Pharmacol Sci 13:236–241
Noda M, Ikeda T, Kayano T, Suzuki K, Takeshima H, Kurasaki M, Takahashi H, Numa S (1986) Existence of distinct sodium channel messenger RNAs in rat brain. Nature 320:188–192
Osborne MP, Pepper DR, Hein PJD (1995) Site-insensitive mechanisms in knockdown resistant strains of house fly larva,Musca domestica. In: Clark JM (ed) Molecular action of insecticides on ion channels. ACS Symposium Series 591. American Chemical Society, Washington DC, pp 128–148
Pauron D, Barhanin J, Amichot M, Pralavorio M, Berge JB, Lazdunski M (1989) Pyrethroid receptor in the insect Na+ channel: alteration of its properties in pyrethroid-resistant flies. Biochemistry 28:1673–1677
Pepper DR, Osborne MP (1993) Electrophysiological identification of site-insensitive mechanisms in knockdown resistant strains of the housefly larva (Musca domestica). Pesticide Sci 39:279–286
Ptacek LJ, George AL, Griggs RC, Tawil R, Kallen RG, Barchi RL, Robertson M, Leppert MF (1991) Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell 67:1021–1027
Ragsdale DS, McPhee JC, Scheuer T, Catterall WA (1994) Molecular determinants of state dependent block of Na+ channels by local anesthetics. Science 265:1724–1728
Rojas CV, Wang JZ, Schwartz LS, Hoffman EP, Powell BR, Brown RH (1991) A Met to Val mutation in the skeletal muscle Na+ channel alpha subunit in hyperkalemic periodic paralysis. Nature 354:387–389
Salkoff L, Butler A, Wei A, Scavarda N, Giffen K, Ifune C, Goodman R, Mandel G (1987) Genomic organization and deduced amino acid sequence of a putative sodium channel gene inDrosophila. Science 237:744–749
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sattelle DB, Leech CA, Lummis CR, Harrison BJ, Robinson HPC, Moores GD, Devonshire AL (1988) Ion channel properties of insects susceptible and resistant to insecticides. In: Lunt GG (ed) Neurotox '88: molecular basis of drug and pesticide action. Elsevier, Amsterdam, pp 563–582
Sawicki RM (1978) Unusual response of DDT-resistant houseflies to carbinol analogues of DDT. Nature 275:443–444
Soderlund DM, Bloomquist JR (1989) Neurotoxic actions of pyrethroid insecticides. Annu Rev Entomol 34:77–96
Soderlund DM, Bloomquist JR (1990) Molecular mechanisms of insecticide resistance. In: Roush RT, Tabashnik BE (eds) Pesticide resistance in arthropods. Chapman and Hall, New York, pp 58–96
Stuhmer W, Conti F, Suzuki H, Wang XD, Noda M, Yahagi N, Kubo H, Numa S (1989) Structural parts involved in activation and inactivation of the sodium channel. Nature 339:597–603
Taylor MFJ, Heckel DG, Brown TM, Kreitman ME, Black B (1993) Linkage of pyrethroid insecticide resistance to a sodium channel locus in the tobacco budworm. Insect Biochem Mol Biol 23:763–775
Thackeray JR, Ganetzky B (1994) Developmentally regulated alternative splicing generates a complex array ofDrosophila para sodium channel isoforms. J Neurosci 14:2569–2578
Vassilev P, Scheuer T, Catterall WA (1989) Inhibition of inactivation of single sodium channels by a site-directed antibody. Proc Natl Acad Sci USA 86:8147–8151
Vijverberg HPM, Ruigt GSF, van den Bercken J (1982) Structure-related effects of pyrethroid insecticides on the lateral line sense organ and on peripheral nerves of the clawed frog,Xenopus laevis. Pesticide Biochem Physiol 18:315–324
Williamson MS, Denholm I, Bell CA, Devonshire AL (1993) Knockdown resistance (kdr) to DDT and pyrethroid insecticides maps to a sodium channel gene locus in the housefly (Musca domestica). Mol Gen Genet 240:17–22
Wu CF, Ganetzky B (1980) Genetic alteration of nerve membrane excitability in temperature sensitive paralytic mutants ofDrosophila melanogaster. Nature 286:814–816
Author information
Authors and Affiliations
Additional information
Communicated by M. Ashburner
Rights and permissions
About this article
Cite this article
Williamson, M.S., Martinez-Torres, D., Hick, C.A. et al. Identification of mutations in the houseflypara-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Molec. Gen. Genet. 252, 51–60 (1996). https://doi.org/10.1007/BF02173204
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02173204