Summary
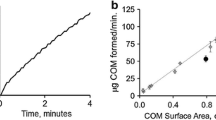
A constant composition method has been used to examine the dissolution kinetics of calcium oxalate renal stones over a wide range of undersaturationin vitro. Demineralization experiments have been carried out with the concentrations of calcium and oxalate ions and ionic strength (hence the solution undersaturation) held constant by the potentiometrically controlled addition of medium electrolyte solution as diluent, triggered by a calcium ion electrode. Kinetic data for renal stones have been compared with results obtained for synthetic calcium oxalate. In addition, constant composition results have been directly compared with results obtained using conventional dissolution methods for both calculi and synthetic calcium oxalate. Overall, calcium oxalate renal stones exhibited markedly different kinetic dissolution behavior as compared with synthetic controls. The renal stone samples dissolved more slowly at all undersaturations, exhibited increased kinetic orders of reaction, and showed reduced sensitivity to solution hydrodynamics. Stones composed of mixed hydrates of calcium oxalate (mono- and di-) came to dihydrate equilibrium in conventional experiments and underwent net dissolution in solutions supersaturated to monohydrate under constant composition conditions. No conversion of di- to monohydrate was observed under these experimental conditions. These results indicate that stone dissolution is strongly influenced by adsorbed inhibitors, presumaly including matrix components, which may complicate efforts to develop systemic and/or irrigation measures effective forin situ solubilization.
Similar content being viewed by others
References
Miller RA, Payne SR, Wickham JEA (1984) Electrohydraulic nephrolithotripsy: a preferable alternative to ultrasound. Br J Urol 56:589–593
Oosterlink W, De Sy WA (1983) A new percutaneous nephrostomy set. J Urol 129:466–467
Kallistratos G (1975) Litholytic agents with bacteriostatic properties in the conservative treatment of urolithiasis. Eur Urol 1:261–269
Dretler SP, Pfister RC (1983) Percutaneous dissolution of renal calculi. Ann Rev Med 34:359–366
Carson CC, Moore AV, Weinerth JL, Ford KK, Reed Dunnick N (1984) Percutaneous dissolution of renal calculi using ultrasonic lithopalaxy. South Med J 77:196–199
Kursh ED, Resnick MI (1984) Dissolution of uric acid calculi with systemic alkalinization. J Urol 132:286–287
Nieh PT, Wurzel RS (1985) Dissolution of uric acid calculi with intravenous 1/6 molar lactate. Urol 26:129–134
Tung KH, Tan EC, Foo KT (1984) Chemolysis of uric acid stones. Ann Acad Med Singapore 13:620–624
Spataro RF, Linke CA, Bartaria ZL (1978) The use of percutaneous nephrostomy and urinary alkalization in the dissolution of obstructing uric acid stones. Diag Radiol 129:629–632
Ansari ER, Kazim E, Husain I (1982) Management of the choked ureter in obstructive renal failure due to uric acid lithiasis. J Urol 128:257–261
Sadi MV, Saltzman N, Feria G, Gittes RF (1985) Experimental observations on dissolution of uric acid calculi. J Urol 134:575–579
Gotz F, Frang D, Hubber J, Nagy Z (1982) Combined oral and local therapy for the dissolution of urinary calculi. Int Urol Nephrol 14:239–246
Dretler SP, Pfister RC (1984) Primary dissolution therapy of struvite calculi. J Urol 131:861–863
Sheldon CA, Smith AD (1982) Chemolysis of calculi. Urol Clin N Am 9:121–130
Verplaetse H, Verbeeck RMH, Minnaert H, Oosterlinck W (1985) Solubility of inorganic kidney stone components in the presence of acid-base sensitive complexing agents. Eur Urol 11:44–51
Verplaetse H, Verbeeck RMH, Minnaert H, Osterlinck W (1986) Screening of chelating agents of chemolysis. Eur Urol 12:190–194
Elliot JS (1973) Structure and composition of urinary calculi. J Urol 109:82–83
Pawelchak JM (1982) Dissolution mechanisms of renal calculi. PhD thesis, University of Connecticut
Pawelchak JM, Flanagan DR, Simonelli AP (1981) Rates and mechanisms of dissolution of renal calculi. I. Rates and mechanisms of dissolution of pure calcium oxalate monohydrate in acid and EDTA solution. In: Smith LH, Robertson WG, Finlayson B (eds) Urolithiasis: clinical and basic research. Plenum Press, New York, pp 539–544
Pawelchak JM, Flanagan DR, Simonelli AP (1981) Rates and mechanisms of dissolution of renal calculi. II. Development and discussion of potential models for dissolution of oxalate calculi. In: Smith LH, Robertson WG, Finlayson B (eds) Urolithiasis: clinical and basic research. Plenum Press, New York, pp 545–549
Pawelchak JM, Flanagan DR, Simonelli AP (1981) Rates and mechanisms of dissolution of renal calculi. III. Mechanisms and rates of dissolution of simulated oxalate calculi in acid and EDTA solutions. In: Smith LH, Robertson WG, Finlayson B (eds) Urolithiasis: clinical and basic reserch. Plenum Press, New York, pp 551–556
Wong WH (1982) Dissolution of calcium oxalate monohydrate and artificial renal calculi in reactive media. PhD Thesis, University of Iowa
Ziolkowski F, Perrin DD (1977) Dissolution of urinary stones by calcium chelating agents—a study using a model system. Invest Urol 15:208–211
Burns JR, Belcher JA, Finlayson B (1985) Dissolution kinetics of calcium oxalate calculi. In: Schwille PD, Smith LH, Robertson WG, Vahlensieck W (eds) Urolithiasis and related clinical research. Plenum Press, New York, pp 757–760
Butkevitch OV, Charkov AK, Panin AG (1982) Dissolution kinetics of calculi in complexone solutions. Vestnik Lening Univ 16:114–117
Butkevitch OV, Charkov AK,Panin AG (1982) Study of the effectiveness of the dissolution processes on calcium oxalate renal stones in various solutions. Vestnik Lening Univ 4:70–75
Butkevitch OV, Charkov AK, Panin AG, Zvinchuk RA (1981) Comparative study of the dissolution processes of phosphate, oxalate and urate renal stones in citrate solutions. Vestnik Lening Univ 10:106–111
Griffith DP, Bragin S, Musker DM (1976) Dissolution of struvite urinary stones—experimental studies in vitro. Invest Urol 13:351–353
Burns JR, Gauthier JF, Finlayson B (1984) Dissolution kinetics of uric acid calculi. J Urol 131:708–711
Tomazic B, Nancollas GH (1980) The kinetics of dissolution of calcium oxalate hydrates. II. The dihydrate. Invest Urol 18:97–101
Tomazic B, Nancollas GH (1980) Crystal growth of calcium oxalate hydrates: a comparative kinetics study. J Coll Int Sci 75:149–159
Tomazic B, Nancollas GH (1979) A study of the phase transformation of calcium oxalate trihydrate-monohydrate. Invest Urol 16:329–335
Tomazic B, Nancollas GH (1982) The dissolution of calcium oxalate kidney stones. A kinetic study. J Urol 128:205–208
White DJ, Nancollas GH (1982) The kinetics of dissolution of calcium oxalate monohydrate. A constant composition study. J Crystal Growth 57:267–272
Nancollas GH, Gardner GL (1974) Kinetics of crystal growth of calcium oxalate monohydrate. J Crystal Growth 21:267–276
Tomson MB, Barone JP, Nancollas GH (1977) Precise calcium phosphate determination. Atomic Absorpt Newslett 16:117–18
Gardner GL, Nancollas GH (1975) Kinetics of dissolution of calcium oxalate monohydrate. J. Phys Chem 79:2597–2600
Nancollas GH (1979) The growth of crystals in solution. Adv Coll Int Sci 10:215–252
Nancollas GH (1966) Interactions in electrolyte solutions. Elsevier, Amsterdam, pp 60–80
Davies CW (1962) Ion association. Butterworths, London
White DJ (1982) A constant composition study of the kinetics of crystallization and demineralization of calcium oxalate: applications to renal stone disease. PhD thesis, SUNY at Buffalo
Tomazic B, Nancollas GH (1979) The kinetics of dissolution of calcium oxalate hydrates. J Crystal Growth 46:355–361
Gaur SS, Nancollas GH (1984) Kinetics of crystal growth in urine. Kid Int 26:767–768
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
White, D.J., Coyle-Rees, M. & Nancollas, G.H. Kinetic factors influencing the dissolution behavior of calcium oxalate renal stones: A constant composition study. Calcif Tissue Int 43, 319–327 (1988). https://doi.org/10.1007/BF02556642
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02556642