Abstract
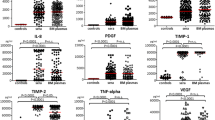
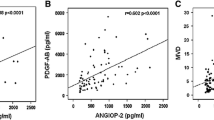
Angiogenesis is a process that plays an important role in the growth and progression of cancer; growing evidence suggests that neovascularization is important in hematologic malignancies. Increased angiogenic potential has been identified in multiple myeloma (MM). In this study, investigators simultaneously measured the levels of hepatocyte growth factor (HGF), platelet-derived growth factor-AB (PDGF-AB), and transforming growth factor-alpha (TGF-/ga) through enzyme-linked immunosorbent assay in the bone marrow (BM) and peripheral blood (PB) of 30 patients with MM and 10 healthy controls. Differences in HGF values in BM sera were significant (P=.001) between patients and controls. In detailed analyses of HGF, PDGF-AB, and TGF-α, according to disease stage, a significant correlation was found between disease stage and BM HGF (P=.047), BM TGF-α (P=.021), and PB PDGF-AB (P=.006), respectively. When correlations between all other parameters were analyzed, significance was noted between PB TGF-α and lactate dehydrogenase (P=.02), PB TGF-α and PB HGF (P=.002), BM TGF-α and CD38 (P=.046), BM TGF-α and BM HGF (P=.000), BM TGF-α and BM PDGF-AB (P=.048), BM HGF and PB HGF (P=.044), and BM PDGF-AB and PB PDGF-AB (P=.000). BM HGF levels had a significant effect on overall survival, with disease severity assessed in terms of disease stage (P=.0018, log-rank test). These data show that in patients with MM, high levels of BM HGF, BM TGF-α, and PB PDGF-AB were associated with advanced disease stage; in addition, HGF played a significant role in disease processing and was related to disease severity. These findings have also led to the concept of a symbiotic relationship between the growth of myeloma cells and HGF, TGF-α, and PDGF-AB in BM.
Similar content being viewed by others
References
Kawano M, Hirano T, Matsuda T, et al. Autocrine generation and requirement of BSF-2/ IL-6 for human multiple myelomas.Nature. 1988;332:83–85.
Anderson KC, Jones RM, Morimoto C, Leavitt P, Barut BA. Response patterns of purified myeloma cells to hematopoietic growth factors.Blood. 1989;73:1915–1924.
Klein B, Zhang XG, Jourdan M, et al. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6.Blood. 1989;73:517–526.
Bataille R, Harousseau JL. Multiple myeloma.N Engl J Med. 1997;336:1657–1664.
Vacca A, Di Loreto M, Ribatti D, et al. Bone marrow of patients with active multiple myeloma: angiogenesis and plasma cell adhesion molecules LFA-1, VLA-4, LAM 1 and CD44.Am J Hematol. 1995;50:9–14.
Vacca A, Ribatti D, Roncali L, et al. Bone marrow angiogenesis and progression in multiple myeloma.Br J Haematol. 1994;87:503–508.
Rajikumar SV, Fonseca R, Witzig TE, Gertz MA, Greipp PR. Bone marrow angiogenesis in patients achieving complete response after stem cell transplantation for multiple myeloma.Leukemia (Baltimore). 1999;13:469–472.
Folkman J, Klagsburn M. Vascular physiology, a family of angiogenic peptides.Nature. 1987;329:671–672.
Schweiger L, Neufield G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth.Nature. 1987;325:257–259.
Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth.J Cell Biol. 1992;119:629–641.
Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility.Nature. 1987;327:239–242.
Borset M, Seidel C, Hjorth-Hansen H, Waage A, Sundan A. The role of hepatocyte growth factor and its receptor c-Met in multiple myeloma and other blood malignancies.Leuk Lymphoma. 1999;32:249–256.
Dankbar B, Padro T, Leo R, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor stromal cell interactions in multiple myeloma.Blood. 2000;95:2630–2636.
Jacobson JL, Hussein MA, Barlogie B, Durie BG, Crowly JJ. Southwest Oncology Group. A new staging system for multiple myeloma patients based on the Southwest Oncology Group (SWOG) experience.Br J Haematol. 2003;122:441–450.
Alexanian R, Balcerzak S, Bonnet JD, et al. Prognostic factors in multiple myeloma.Cancer. 1975;36:1192–1201.
Zhan F, Hardin J, Kordsmeier B, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells.Blood. 2002;99:1745–1757.
Galimi F, Bagnara GP, Bonsi L, et al. Hepatocyte growth factor induces proliferation and differentiation of multipotent and erythroid hemopoietic progenitors.J Cell Biol. 1994;127:1743–1754.
Weimar IS, Miranda N, Muller EJ, et al. Hepatocyte growth factor/scatter factor (HGF/SF) is produced by human bone marrow stromal cells and promotes proliferation, adhesion and survival of human hematopoietic progenitor cells (CD34+).Exp Hematol. 1998;26:885–894.
Weimar IS, Voermans C, Bourhis JH, et al. Hepatocyte growth factor/scatter factor (HGF/SF) affects proliferation and migration of myeloid leukemic cells.Leukemia. 1998;12:1195–1203.
Borset M, Lien E, Espevik T, Helseth E, Waage A, Sundan A. Concomitant expression of hepatocyte growth factor/scatter factor and the receptor c-MET in human myeloma cell lines.J Biol Chem. 1996;271:24655–24661.
Ribatti D, Vacca A, Nico B, et al. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma.Br J Cancer. 1999;79:451–455.
Vacca A, Ribatti D, Presta M, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma.Blood. 1999;93:3064–3073.
Morimoto A, Okamura K, Hamanaka R, et al. Hepatocyte growth factor modulates migration and proliferation of human microvascular endothelial cells in culture.Biochem Biophys Res Commun. 1991;179:1042–1049.
Rosen EM, Grant D, Kleinman H, et al. Scatter factor stimulates migration of vascular endothelium and capillary-like tube formation.Cell Motil Factors. 1991;59:76–88.
Morimoto A, Tada K, Nakayama Y, et al. Cooperative roles of hepatocyte growth factor and plasminogen activator in tubular morphogenesis by human microvascular endothelial cells.Jpn J Cancer Res. 1994;85:53–62.
Grant DS, Kleinman HK, Goldberg ID, et al. Scatter factor induces blood vessel formation in vivo.Proc Natl Acad Sci USA. 1993;90:1937–1941.
Rosen EM, Grant DS, Kleinman HK, et al. Scatter factor (hepatocyte growth factor) is a potent angiogenesis factor in vivo.Symp Soc Exp Biol. 1993;47:227–234.
Shin DM, Ro JY, Hong WK, Hittelman WN. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis.Cancer Res. 1994;54:3153–3159.
Santini J, Formento JL, Francoual M, et al. Characterisation, quantification, and potential clinical value of epidermal growth factor receptor in head and neck squamous cell carcinomas.Head Neck. 1991;13:132–139.
Dassonville O, Formento JL, Francoual M, et al. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer.J Clin Oncol. 1993;11:1873–1878.
Rubin Grandis J, Tweardy DJ, Melhem MF. Asynchronous modulation of transforming growth factor-α and epidermal growth factor receptor protein expression in progression of premalignant lesions to head and neck squamous cell carcinoma.Clin Cancer Res. 1998;4:13–20.
Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis.Science. 1973;180:1332–1339.
Josephs SF, Ratner L, Clarke MF, Westin EH, Reitz MS, Wong-Staal F. Transforming potential of human c-sis nucleotide sequences encoding platelet-derived growth factor.Science. 1984;225:636–639.
Hannink M, Donoghue DJ. Structure and function of platelet-derived growth factor (PDGF) and related proteins.Biochim Biophys Acta. 1989;989:1–10.
Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits.Bone. 1994;15:203–208.
Mitlak BH, Finkelman RD, Hill EL, et al. The effect of systemically administered PDGF-BB on the rodent skeleton.J Bone Miner Res. 1996;11:238–247.
Cohen AM, Soderberg C, Thomason A. Plasma clearance and tissue distribution of recombinant human platelet-derived growth factor (B-chain homodimer) in rats.J Surg Res. 1990;49:447–452.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kara, I.O., Sahin, B., Gunesacar, R. et al. Clinical significance of hepatocyte growth factor, platelet-derived growth factor-AB, and transforming growth factor-α in bone marrow and peripheral blood of patients with multiple myeloma. Adv Therapy 23, 635–645 (2006). https://doi.org/10.1007/BF02850052
Issue Date:
DOI: https://doi.org/10.1007/BF02850052