Summary
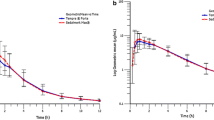
The pharmacokinetics of a new tablet formulation of acenocoumarol racemate, an oral anticoagulant agent, has been investigated in 8 normal healthy subjects. The drug was given as a single oral dose of 12 mg. 12 blood samples were collected after administration. Plasma acenocoumarol concentrations were determined by a sensitive HPLC method. Areas under the plasma level-time curves for each subject were evaluated by means of the trapezoidal rule. The peak plasma concentration of 244.19–644.23 μg/l was reached 1–4 h after drug administration. The terminal phase half-life was 6.29–14.22 h and a systemic clearance was 1.86–5.62 l/h. The new tablet formulation of acenocoumarol seems to be bioequivalent when compared to the one used so far. For the prediction of systemic availability and estimation of the first-pass metabolism, from plasma level data, a hepatic blood flow rate limited model were used. The systemic availability was 94.22–98.01% and the elimination of the drug on its first-pass through the liver was 1.99–5.78%.
Similar content being viewed by others
References
Godbillon J., Richard J., Gerardin A., Meinertz T., Kasper W., Jahnchen E. (1981): Pharmacokinetics of the enantiomers of acenocoumarol in man. Br. J. Clin. Pharmacol., 12, 621–629.
Dieterle W., Faigle J.W., Montigel C., Sulc M., Theobald W. (1977): Biotransformation and pharmacokinetics of acenocoumarol (Sintrom) in man. Eur. J. Clin. Pharmacol., 11, 367–375.
Thijssen H.H.W., Baars L.G. (1983): Active metabolites of acenocoumarol: do they contribute to the therapeutic effect? Br. J. Clin. Pharmacol., 16, 491–496.
Thijssen H.H.W., Baars L.G., Reijnders M.J. (1983): Analysis of acenocoumarin and its amino and acetamido metabolites in body fluids by high-performance liquid chromatography. J. Chromatogr., 274, 231–238.
de Wolff F.A., Tetteroo-Tempelman C.A.M., Edelbroek P.M. (1980): Determination of nanogram levels of the anticoagulant acenocoumarin in serum by high-performance liquid chromatography. J. Anal. Toxicol., 4, 156–159.
Gibaldi M., Boyes R.N., Feldman S. (1971): Influence of first-pass effect on availability of drugs on oral administration. J. Pharm. Sci., 60, 1338–1340.
Perrier D., Gibaldi M., Boyes R.N. (1973): Prediction of systemic availability from plasma-level data after oral drug administration. J. Pharm. Pharmacol., 25, 256–257.
Gibaldi M., Perrier D. (1975): Pharmacokinetics. New York, Marcel Dekker.
Vaughan D.P. (1975): Estimation of biological availability after drug administration when the drug is eliminated by urinary excretion and metabolism. J. Pharm. Pharmacol., 27, 458–461.
McLean A.J., McNamara P.J., duSouich P., Gibaldi M., Lalka D. (1978): Food, splanchnic blood flow, and bioavailability of drugs subject to first-pass metabolism. Clin. Pharmacol. Ther., 24, 5–10.
Popović J. (1985): Estimation of the first-pass metabolism of a drug during multiple oral dosage. Periodicum Biologorum, 87, 290–292.
Popović J. (1985): Influence of first-pass effect on availability of drugs with simultaneous biotransformation in the liver and first-order elimination through kidneys. Yugosl. Physiol. Pharmacol. Acta, 21 (Suppl. 3), 289–290.
Popović J. (1986): Dosage regimen calculations for drugs with first-order absorption, non-linear first-pass metabolism and parallel non-linear and first-order elimination. Periodicum Biologorum, 88, 183–184.
Popović J. (1987): Relationship between the steady-state serum level and the dose of drugs with first-pass and parallel Michaelis-Menten and first-order elimination. Acta Pharm. Jugosl., 37, 313–317.
Balant L.P., Benet L.Z., Blume H., Bozler G., Breimer D.D., Eichelbaum M. (1991): Is there a need for more precise definitions of bioavailability? Eur. J. Clin. Pharmacol., 40, 123–126.
Salmonson T., Rane A. (1990): Clinical pharmacokinetics in the drug regulatory process. Clin. Pharmacokinet., 18, 177–183.
O’Reilly R.A. (1974): Studies on the optical enantiomorphs of warfarin in man. Clin. Pharmacol. Ther., 16, 348–354.
Selers E.M., Koch-Weser J. (1975): Interaction of warfarin stereoisomers with human albumin. Pharmacol. Res. Commun., 7, 331–336.
Hewick D.S., Shepard A.M.M. (1976): The plasma elimination of the enantiomers of phenprocoumon in man. J. Pharm. Pharmacol., 28, 257–258.
Jahnchen E., Meinertz T., Gilfrich H.J., Groth U., Martini A. (1976): The enantiomers of phenprocoumon. Pharmacodynamic and pharmacokinetic studies. Clin. Pharmacol. Ther., 20, 342–349.
Kelly J.G., O’Malley K. (1979): Clinical pharmacokinetics of oral anticoagulants. Clin. Pharmacokinet., 4, 1–15.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Popović, J., Mikov, M. & Jakovljević, V. Pharmacokinetic analysis of a new acenocoumarol tablet formulation during a bioequivalence study. Eur. J. Drug Metab. Pharmacokinet. 19, 85–89 (1994). https://doi.org/10.1007/BF03188828
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03188828