Summary
The pharmacokinetics and biochemical efficacy of N-(2-memyl-2-propyl)-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide (I) were evaluated in healthy male volunteers after single and multiple oral administration.
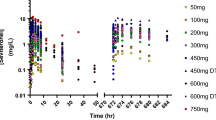
The mean times to reach peak plasma concentrations (Tmax) of (I) at doses of 5, 10, 20, SO and 100 mg ranged from 1.8 to 2.8 h, and the corresponding mean peak plasma concentrations (Cmax) were 38.1, 81.5, 147.1, 442.0 and 835.5 ng/ml, respectively.
The drug disappeared from the systemic circulation with half-lives (t1/2) of 4.7–7.1 h. The mean values of the area under the curve (AUC0–24) and Cmax increased linearly in a dose-dependent manner.
After multiple oral adniinistration of (I) (10 mg/d) for 7 days, Cnua and AUC increased slightly during administration, however, there were no significant differences between day 4 and day 7. Although there were large intersubject differences in the 24 h plasma levels after each dosing, no accumulation of (I) occurred after 7 days dosing.
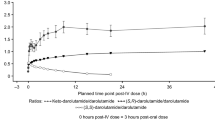
Serum 5-α-dihydrotestosterone (DHT) was markedly reduced at all dose levels. The mean serum levels of DHT at 24 h post-dosing decreased to 27–42% of that before dosing. On the other hand, the serum testosterone (T) did not change significantly. After multiple administration of (I), serum DHT was significantly reduced and remained suppressed for up to 7 days after the final dosing.
Similar content being viewed by others
References
Rasmusson G.H., Reynolds G.F., Utne T., et al. (1984): Azasteroids as inhibitors of rat prostatic Sa-reductase. J. Med. Chem., 27, 1690–1701.
Liang T., Cascieri M.A., Cheung A.H., Reynolds G.F., Rasmusson G.H. (1985): Species difference in prostatic steroids 5α-reductases of rat, dog and human. Endocrinology, 117, 571–579.
Brooks J.R., Berman C., Primka R.L., Reynolds G.F., Rasmusson G.H. (1986): 5α-Reductase inhibitory and anti-androgenic activities of some 4-azasteroids in the rat. Steroids, 47, 1–19.
Brooks J.R., Baptiska E.M., Berman C., et al. (1981): Response of rat ventral prostate to a new and novel 5α-reductase inhibitor. Endocrinology, 109, 830–836.
Wenderoth U.K., George F.W., Wilson J.D. (1983): The effect of a 5α-reductase inhibitor on androgen-mediated growth of the dog prostate. Endocrinology, 113, 569–573.
Brooks J.R., Berman C., Glitzer M.S., et al. (1982): Effect of a new Sa-reductase inhibitor on size, histologie characteristics and androgen concentrations of the canine prostate. Prostate, 3, 35–44.
Siiteri P.K., Wilsom J.D. (1970): Dihydrostestosterone in prostatic hypertrophy I. The formation and content of dihydrotestosterone in the hypertrophic prostate of man. J. Clin. Invest., 49, 1737–1745.
Peters C., Scott W.W., Walsh P.C. (1986): The response of human BPH to androgen deprivation. J. Urol., 135, 196A.
Geller J., Albert J., Lopez D., Geller S., Niwayama G. (1976): Comparison of androgen metabolites in benign prostatic hypertrophy and normal prostate. J. Clin. Endocrinol. Metab., 43, 686–688.
Carlin J.R., Christofalo P., Vandenheuvel J.A. (1988): High-performance liquid chromatographic determination of N-(2-methyl-2-propyl)-3-oxo-4-aza-5α-androst-1 -ene- 17β-carbox amide, a 4-azasteroid in human plasma from a phase I study. J. Chromatogr., 427, 79–91.
Kawamura J., Okada K., Yoshida O., Kousaka T., Torizuka K. (1986): Testosterone radioimmunoassay with125I-testosterone kit. Kakuigaku, 23, 431–438.
Auletta F.J., Caldwell B.V., Hamilton G.L. (1974): Methods of hormone radioimmunoassay. Jaffe B.M., Behrman R.H. eds. New York, Academic Press, pp. 359–370.
Gibaldi M., Perrier D. (1982): In: Swarbrick J. ed, Pharmacokinetics. New York, Marcel Decker, pp. 149–152.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohtawa, M., Morikawa, H. & Shimazaki, J. Pharmacokinetics and biochemical efficacy after single and multiple oral administration of N-(2-methyl-2-propyl)-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide, a new type of specific competitive inhibitor of testosterone 5α-reductase, in volunteers. European Journal of Drug Metabolism and Pharmacokinetics 16, 15–21 (1991). https://doi.org/10.1007/BF03189869
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189869