Summary
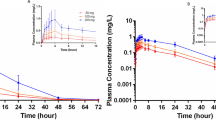
In a randomized 2-way cross-over study with twelve healthy male volunteers, two colchicine preparations (tablets, A vs. oral solution, B) were tested. The preparations were administered as single doses of 1 mg; prior to and up to 72 h after medication blood samples were collected and the plasma colchicine concentrations determined. Additionally urine samples were collected at 0–2, 2–4, 4–6, 6–8, 8–10, 10–24, 24–48, 48–72 and 72–96 h intervals. The colchicine plasma and urine concentrations were determined by a newly developed and validated RIA method. The mean area under the plasma concentration-time curve (AUC-1, AUC-3) was calculated as 23.95±12.10 (AUC-1) and 26.73±12.75 (AUC-3) h.ng/ml after application of A and 28.01±14.74 (AUC-1) and 31.57+16.58 (AUC-3) h.ng/ml after application of B, respectively. Mean peak plasma concentrations of 4.15±2.35 (A) and 4.88±3.90 (B) ng/ml were reached at 1.15±0.38 (A) and 1.13±0.42 (B) h after application. The mean terminal half-lives accounted for 9.31±3.98 (A) and 10.57±5.53 (B) h. The mean total clearance (Cl/F) and volume of distribution (V/F) were found to be 40.12±20.87 (A) and 46.58±24.65 (B) 1/h and 472.59±196.46 (A) and 624.89±304.09 (B) 1, respectively. The mean total amount excreted in urine (Ae) was 172.66±91.51 (A) and 174.85±63.53 (B) ug. The statistical evaluations of the pharmacokinetic parameters showed no significant differences, either for the extent of absorption (as indicated by the AUQ, or for the rate of absorption (as indicated by Cmax and tmax) between both preparations.
The relative bioavailability (assuming the solution to be 100%) accounted for 115% (AUC-1) and 114% (AUC-3), as calculated from the plasma data, and 121% as calculated from the urine data, respectively. The present results show that no statistically significant differences between the preparations exist for the relevant pharmacokinetic parameters (AUC-1, AUC-3, Cmax, tmax, Ae).
Similar content being viewed by others
References
Drag Information 89™ (1989): American Hospital Formulary Service®, American Society of Hospital Pharmacists, Inc. Bethesda, MD 20814, USA.
Wellhöner H-H. (1982): Pharmakologie und Toxikologie, 3. Aufl. Berlin, Springer Verlag.
Product Profile Colchicum-Dispert®, Kali-Chemie Pharma GmbH, D-3000 Hannover, FRG.
Mutschier E. (1981): Arzneimittelwirkungen, Wissenschani., Verlagsgesellschaft, Stuttgart, FRG.
Klotz U. (1984): Klinische Pharmakokinetik, G. Fischer Verlag, Stuttgart, FRG.
DAZ Giftlexikon ‘Colchicin’ (1988): Dt. Apoth. Ztg., 128, 693–697.
Scherrmann J.M., Boudet L., Pontikis R., Nam, N.H., Foumier E. (1980): A sensitive radioimmunoassay for colchicine, J. Pharm. Pharmacol., 32, 800–802.
APV Richtlinie ‘Untersuchungen zur Bioverfügbarkeit, Bioäquivalenz’ (1987): Pharm. Ind., 49, 704–707.
Katz E.Z., Ehrenfeld M., Levy M., Eliakim M. (1982): Plasma colchicine concentration in patients with recurrent polyserositis (familial Mediterranean fever) on long-term prophylaxis. Arthritis Rheum., 25, 227–231.
Halkin H., Dany S., Greenwald M., Shnaps Y., Tirosh M. (1980): Colchicine kinetics in patients with familial Mediterranean fever. Clin. Pharmacol. Ther., 28, 82–87.
Ertel N.H.,Mittler J.C., Akgun S., Wallace S.L. (1976): Radioimmunoassay for colchicine in plasma and urine. Science, 193, 233–235.
Creasey W.A., Bensh K.G., Malawista S.E. (1971): Colchicine, Vinblastine and Griseoftilvin. Pharmacological studies with human leucocytes. Biochem. Pharmacol., 20, 1579–1588.
Ertel N.H., Wallace S.L. (1971): Measurement of colchicine in urine and peripheral leucocytes. Clin. Res., 19, 348.
Hunter A.L., Klaassen C.D. (1975): Biliary excretion of colchicine. J. Pharmacol. Exp. Ther., 192, 605–617
Author information
Authors and Affiliations
Additional information
The clinical part of the study was performed at: LAB GmbH & Co., Wegenerstr. 13, D-7910 Neu-Ulm, FRG
Rights and permissions
About this article
Cite this article
Achtert, G., Scherrmann, J.M. & Christen, M.O. Pharmacokinetics / bioavailability of colchicine in healthy male volunteers. Eur. J. Drug Metab. Pharmacokinet. 14, 317–322 (1989). https://doi.org/10.1007/BF03190118
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190118