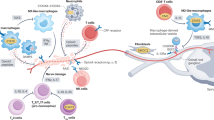
Abstract
Ability to perceive physiological pain is essential in protecting the individual from tissue destruction. In contrast, pathological chronic pain is an expression of maladaptive alterations outlasting its biological usefulness. In such conditions even eating, speaking or wearing clothes might be painful, as in neuropathic pain. Such pain is caused by diseases or injuries affecting nerves (e.g. diabetes, trigeminal neuralgia or amputation). Neuropathic pain is not an exclusive neuronal phenomenon but also involves immune responses. Damaged peripheral nerves are infiltrated by mast cells, granulocytes, macrophages and T lymphocytes. It is widely emphasized that these cells, via secretion of inflammatory mediators (e.g. proinflammatory cytokines, chemokines), contribute to the generation of neuropathic pain. However, leukocytes are also a source of analgesic mediators such as anti-inflammatory cytokines and opioid peptides. Recent findings indicate that immune cell-derived opioid peptides can interact with opioid receptors in the injured nerves and ameliorate neuropathic pain. Targeting opioid-containing immune cells might represent a new disease-modifying approach based on the use of beneficial effects of neuro-inflammation in painful neuropathies. This review analyzes both detrimental and advantageous actions of leukocytes at peripheral nerves in neuropathic pain.

Similar content being viewed by others
Abbreviations
- CCI:
-
Chronic constriction injury
- CFA:
-
Complete Freund’s adjuvant
- CGRP:
-
Calcitonin gene-related peptide
- CNS:
-
Central nervous system
- CRF:
-
Corticotropin-releasing factor
- CXCL1:
-
Chemokine (C-X-C motif) ligand 1 (keratinocyte-derived chemokine)
- CXCL2/3:
-
Chemokine (C-X-C motif) ligand 2 (macrophage inflammatory protein-2)
- CXCR2:
-
CXC chemokine receptor 2
- DRG:
-
Dorsal root ganglion
- ICAM-1:
-
Intercellular adhesion molecule-1
- IL:
-
Interleukin
- KO:
-
Knock-out
- PSL:
-
Partial sciatic nerve ligation
- RAG-1:
-
Recombination-activating gene-1
- SCID:
-
Severe combined immunodeficiency
- SNL:
-
Spinal nerve ligation
- TNF-α:
-
Tumor necrosis factor-α
- TRP:
-
Transient receptor potential ion channels
- WT:
-
Wild type
References
Akins PT, McCleskey EW (1993) Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience 56:759–769
Barclay J, Clark AK, Ganju P et al (2007) Role of the cysteine protease cathepsin S in neuropathic hyperalgesia. Pain 130:225–234
Baron R (2006) Mechanisms of disease: neuropathic pain—a clinical perspective. Nat Clin Pract Neurol 2:95–106
Baron R, Schwarz K, Kleinert A et al (2001) Histamine-induced itch converts into pain in neuropathic hyperalgesia. Neuroreport 12:3475–3478
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Benoliel R, Epstein J, Eliav E et al (2007) Orofacial pain in cancer. Part I. Mechanisms. J Dent Res 86:491–505
Binder W, Mousa SA, Sitte N et al (2004) Sympathetic activation triggers endogenous opioid release and analgesia within peripheral inflamed tissue. Eur J Neurosci 20:92–100
Börzsei R, Pozsgai G, Bagoly T et al (2008) Inhibitory action of endomorphin-1 on sensory neuropeptide release and neurogenic inflammation in rats and mice. Neuroscience 152:82–88
Brack A, Labuz D, Schiltz A et al (2004a) Tissue monocytes/macrophages in inflammation: hyperalgesia versus opioid-mediated peripheral antinociception. Anesthesiology 101:204–211
Brack A, Rittner HL, Machelska H et al (2004b) Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain 112:229–238
Bradbury J (2003) Beyond pills and jabs. Researchers develop new ways to get drugs to the right place at the right time. Lancet 362:1984–1985
Busch-Dienstfertig M, Stein C (2010) Opioid receptors and opioid peptide-producing leukocytes in inflammatory pain: basic and therapeutic aspects. Brain Behav Immun 24:683–694
Cabot PJ, Carter L, Gaiddon C et al (1997) Immune cell-derived β-endorphin: production, release and control of inflammatory pain in rats. J Clin Invest 100:142–148
Cabot PJ, Carter L, Schäfer M et al (2001) Methionine-enkephalin-and Dynorphin A-release from immune cells and control of inflammatory pain. Pain 93:207–212
Campbell JN, Meyer RA (2006) Mechanisms of neuropathic pain. Neuron 52:77–92
Cao L, DeLeo JA (2008) CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol 38:448–458
Childs EA, Lyles RH, Selnes OA et al (1999) Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology 52:607–613
Chizhmakov I, Yudin Y, Mamenko N et al (2005) Opioids inhibit purinergic nociceptors in the sensory neurons and fibres of rat via a G protein-dependent mechanism. Neuropharmacology 48:639–647
Clatworthy AL, Illich PA, Castro GA et al (1995) Role of peri-axonal inflammation in the development of thermal hyperalgesia and guarding behavior in a rat model of neuropathic pain. Neurosci Lett 184:5–8
Costigan M, Moss A, Latremoliere A et al (2009a) T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci 29:14415–14422
Costigan M, Scholz J, Woolf CJ (2009b) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32
Cox JJ, Reimann F, Nicholas AK et al (2006) An SCN9A channelopathy causes congenital inability to experience pain. Nature 444:894–898
Cui JG, Holmin S, Mathiesen T et al (2000) Possible role of inflammatory mediators in tactile hypersensivity in rat models of mononeuropathy. Pain 88:239–248
Dowdall T, Robinson I, Meert TF (2005) Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav 80:93–108
Endres-Becker J, Heppenstall PA, Mousa SA et al (2007) Mu-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol Pharmacol 71:12–18
Fields H (2004) State-dependent opioid control of pain. Nat Rev Neurosci 5:565–575
Flatters SJ, Fox AJ, Dickenson AH (2003) Spinal interleukin-6 (IL-6) inhibits nociceptive transmission following neuropathy. Brain Res 984:54–62
Flatters SJ, Fox AJ, Dickenson AH (2004) Nerve injury alters the effects of interleukin-6 on nociceptive transmission in peripheral afferents. Eur J Pharmacol 484:183–191
Fromont A, De Seze J, Fleury MC et al (2009) Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine 45:55–57
George A, Marziniak M, Schäfers M et al (2000) Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-alpha, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain 88:267–275
Gold MS, Levine JD (1996) DAMGO inhibits prostaglandin E2-induced potentiation of a TTX-resistant Na+ current in rat sensory neurons in vitro. Neurosci Lett 212:83–86
Grothe C, Heese K, Meisinger C et al (2000) Expression of interleukin-6 and its receptor in the sciatic nerve and cultured Schwann cells: relation to 18-kD fibroblast growth factor-2. Brain Res 885:172–181
Herbert MK, Just H, Schmidt RF (2001) Histamine excites groups III and IV afferents from the cat knee joint depending on their resting activity. Neurosci Lett 305:95–98
Heumann R, Lindholm D, Bandtlow C et al (1987) Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci USA 84:8735–8739
Honore P, Wade CL, Zhong C et al (2006) Interleukin-1alpha/beta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Brain Res 167:355–364
Hsieh GC, Chandran P, Salyers AK et al (2010) H4 receptor antagonism exhibits anti-nociceptive effects in inflammatory and neuropathic pain models in rats. Pharmacol Biochem Behav 95:41–50
Hua XY, Chen P, Fox A et al (1996) Involvement of cytokines in lipopolysaccharide-induced facilitation of CGRP release from capsaicin-sensitive nerves in the trachea: studies with interleukin-1beta and tumor necrosis factor-alpha. J Neurosci 16:4742–4748
Hua S, Hermanussen S, Tang L et al (2006) The neural cell adhesion molecule antibody blocks cold water swim stress-induced analgesia and cell adhesion between lymphocytes and cultured dorsal root ganglion neurons. Anesth Analg 103:1558–1564
Ingram SL, Williams TJ (1994) Opioid inhibition of Ih via adenylyl cyclise. Neuron 13:179–186
Kabli N, Cahill CM (2007) Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain 127:84–93
Kashiba H, Fukui H, Morikawa Y et al (1999) Gene expression of histamine H1 receptor in guinea pig primary sensory neurons: a relationship between H1 receptor mRNA-expressing neurons and peptidergic neurons. Brain Res Mol Brain Res 66:24–34
Kerschensteiner M, Gallmeier E, Behrens L et al (1999) Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189:865–870
Kiefer R, Kieseier BC, Stoll G et al (2001) The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol 64:109–127
Kim SH, Chung JM (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50:355–363
Kim KJ, Yoon YW, Chung JM (1997) Comparison of three rodent neuropathic pain models. Exp Brain Res 113:200–206
Kirsch M, Campos Friz M, Vougioukas VI et al (2009) Wallerian degeneration and axonal regeneration after sciatic nerve crush are altered in ICAM-1-deficient mice. Cell Tissue Res 338:19–28
Kleinschnitz C, Hofstetter HH, Meuth SG et al (2006) T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp Neurol 200:480–485
Kohno T, Ji RR, Ito N et al (2005) Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain 117:77–87
Kolesnikov Y, El-Maarouf A, Rutishauser U et al (2007) Reorganization of dorsal root ganglion neurons following chronic sciatic nerve constriction injury: correlation with morphine and lidocaine analgesia. Eur J Pharmacol 568:124–133
La Rana G, Russo R, D’Agostino G et al (2008) AM404, an anandamide transport inhibitor, reduces plasma extravasation in a model of neuropathic pain in rat: role for cannabinoid receptors. Neuropharmacology 54:521–529
Labuz D, Berger S, Mousa SA et al (2006) Peripheral antinociceptive effects of exogenous and immune cell-derived endomorphins in prolonged inflammatory pain. J Neurosci 6:4350–4358
Labuz D, Schmidt Y, Schreiter A et al (2009) Immune cell-derived opioids protect against neuropathic pain in mice. J Clin Invest 119:278–286
Labuz D, Schreiter A, Schmidt Y et al (2010) T lymphocytes containing beta-endorphin ameliorate mechanical hypersensitivity following nerve injury. Brain Behav Immun 24:1045–1053
Leonard G, Goffaux P, Mathieu D et al (2009) Evidence of descending inhibition deficits in atypical but not classical trigeminal neuralgia. Pain 147:217–223
Li JL, Ding YQ, Li YQ et al (1998) Immunocytochemical localization of mu-opioid receptor in primary afferent neurons containing substance P or calcitonin gene-related peptide. A light and electron microscope study in the rat. Brain Res 794:347–352
Likar R, Mousa SA, Steinkellner H et al (2007) Involvement of intra-articular corticotropin-releasing hormone in postoperative pain modulation. Clin J Pain 23:136–142
Lindenlaub T, Teuteberg P, Hartung T et al (2000) Effects of neutralizing antibodies to TNF-alpha on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res 866:15–22
Liu T, van Rooijen N, Tracey DJ (2000) Depletion of macrophages reduces axonal degeneration and hyperalgesia following nerve injury. Pain 86:25–32
Ma W, Quirion R (2005) Up-regulation of interleukin-6 induced by prostaglandin E from invading macrophages following nerve injury: an in vivo and in vitro study. J Neurochem 93:664–673
Machelska H (2007) Targeting of opioid-producing leukocytes for pain control. Neuropeptides 41:285–293
Machelska H, Cabot PJ, Mousa SA et al (1998) Pain control in inflammation governed by selectins. Nat Med 4:1425–1428
Machelska H, Mousa SA, Brack A et al (2002) Opioid control of inflammatory pain regulated by intercellular adhesion molecule-1. J Neurosci 22:5588–5596
Machelska H, Schopohl JK, Mousa SA et al (2003) Different mechanisms of intrinsic pain inhibition in early and late inflammation. J Neuroimmunol 141:30–39
Machelska H, Brack A, Mousa SA et al (2004) Selectins and integrins but not platelet-endothelial cell adhesion molecule-1 regulate opioid inhibition of inflammatory pain. Br J Pharmacol 142:772–780
Metcalfe DD (2008) Mast cells and mastocytosis. Blood 112:946–956
Miller RJ, Jung H, Bhangoo SK et al (2009) Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 194:417–449
Milligan ED, Sloane EM, Langer SJ et al (2006) Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 126:294–308
Minami M, Maekawa K, Yabuuchi K et al (1995) Double in situ hybridization study on coexistence of mu-, delta- and kappa-opioid receptor mRNAs with preprotachykinin A mRNA in the rat dorsal root ganglia. Brain Res Mol Brain Res 30:203–210
Mizumura K, Koda H, Kumazawa T (2000) Possible contribution of protein kinase C in the effects of histamine on the visceral nociceptor activities in vitro. Neurosci Res 37:183–190
Moalem G, Tracey DL (2006) Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 51:240–264
Moalem G, Xu K, Yu L (2004) T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 129:767–777
Mousa SA, Zhang Q, Sitte N et al (2001) Beta-Endorphin-containing memory-cells and mu-opioid receptors undergo transport to peripheral inflamed tissue. J Neuroimmunol 115:71–78
Mousa SA, Shakibaei M, Sitte N et al (2004) Subcellular pathways of beta-endorphin synthesis, processing, and release from immunocytes in inflammatory pain. Endocrinology 145:1331–1341
Mousa SA, Straub RH, Schäfer M et al (2007) Beta-endorphin, Met-enkephalin and corresponding opioid receptors within synovium of patients with joint trauma, osteoarthritis and rheumatoid arthritis. Ann Rheum Dis 66:871–879
Murphy PG, Ramer MS, Borthwick L et al (1999) Endogenous interleukin-6 contributes to hypersensitivity to cutaneous stimuli and changes in neuropeptides associated with chronic nerve constriction in mice. Eur J Neurosci 11:2243–2253
Nilsen KB, Nicholas AK, Woods CG et al (2009) Two novel SCN9A mutations causing insensitivity to pain. Pain 143:155–158
Nyland H, Matre R, Mørk S (1981) Immunological characterization of sural nerve biopsies from patients with Guillain–Barré syndrome. Ann Neurol 9(suppl):80–86
Perkins NM, Tracey DJ (2000) Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience 101:745–757
Przewlocki R, Przewlocka B (2005) Opioids in neuropathic pain. Curr Pharm Des 11:3013–3025
Ramer MS, French GD, Bisby MA (1997) Wallerian degeneration is required for both neuropathic pain and sympathetic sprouting into the DRG. Pain 72:71–78
Rapalino O, Lazarov-Spiegler O, Agranov E et al (1998) Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med 4:814–821
Rashid MH, Inoue M, Toda K et al (2004) Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther 309:380–387
Rittner HL, Labuz D, Schaefer M et al (2006) Pain control by CXCR2 ligands through Ca2+-regulated release of opioid peptides from polymorphonuclear cells. FASEB J 20:2627–2629
Rittner HL, Lux C, Labuz D et al (2007) Neurokinin-1 receptor antagonists inhibit the recruitment of opioid-containing leukocytes and impair peripheral antinociception. Anesthesiology 107:1009–1017
Rittner HL, Hackel D, Voigt P et al (2009) Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils. PLoS Pathog 5:e1000362
Rutkowski MD, Pahl JL, Sweitzer S et al (2000) Limited role of macrophages in generation of nerve injury-induced mechanical allodynia. Physiol Behav 71:225–235
Schäfers M, Geis C, Brors D et al (2002) Anterograde transport of tumor necrosis factor-alpha in the intact and injured rat sciatic nerve. J Neurosci 22:536–545
Schäfers M, Geis C, Svensson CI et al (2003a) Selective increase of tumor necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci 17:791–804
Schäfers M, Lee DH, Brors D, Yaksh TL, Sorkin LS (2003b) Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci 23:3028–3038
Scholz J, Woolf CJ (2002) Can we conquer pain? Nat Neurosci 5:1062–1067
Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10:1361–1368
Seltzer Z, Dubner R, Shir Y (1990) A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43:205–218
Shamash S, Reichert F, Rotshenker S (2002) The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci 22:3052–3060
Shubayev VI, Myers RR (2001) Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J Neuroimmunol 114:48–56
Shubayev VI, Angert M, Dolkas J et al (2006) TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci 31:407–415
Simmons DL (2005) Anti-adhesion therapies. Curr Opin Pharmacol 5:398–404
Smith FM, Haskelberg H, Tracey DJ et al (2007) Role of histamine H3 and H4 receptors in mechanical hyperalgesia following peripheral nerve injury. Neuroimmunomodulation 14:317–325
Sommer C, Schäfers M (1998) Painful mononeuropathy in C57BL/Wld mice with delayed Wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res 784:154–162
Sommer C, Marziniak M, Myers RR (1998) The effect of thalidomide treatment on vascular pathology and hyperalgesia caused by chronic constriction injury of rat nerve. Pain 74:83–91
Sommer C, Petrausch S, Lindenlaub T et al (1999) Neutralizing antibodies to interleukin 1-receptor reduce pain associated behavior in mice with experimental neuropathy. Neurosci Lett 270:25–28
Sommer C, Schäfers M, Marziniak M et al (2001) Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst 6:67–72
Sorkin LS, Xiao WH, Wagner R et al (1997) Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 81:255–262
Stein C, Kopf A (2009) Anesthesia and treatment of chronic pain. In: Miller RD (ed) Miller’s anesthesia, 7th edn. Churchill Livingstone, Philadelphia, pp 1797–1818
Stein C, Lang LJ (2009) Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol 9:3–8
Stein C, Zöllner C (2009) Opioids and sensory nerves. Handb Exp Pharmacol 194:495–518
Stein C, Hassan AH, Przewlocki R et al (1990) Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci USA 87:5935–5939
Stein C, Hassan AH, Lehrberger K et al (1993) Local analgesic effect of endogenous opioid peptides. Lancet 342:321–324
Stein C, Schäfer M, Machelska H (2003) Attacking pain at its source: new perspectives on opioids. Nat Med 9:1003–1008
Stremmel C, Horn C, Eder S et al (2005) The impact of immunological parameters on the development of phantom pain after major amputation. Eur J Vasc Endovasc Surg 30:79–82
Tegeder I, Geisslinger G (2004) Opioids as modulators of cell death and survival—unraveling mechanisms and revealing new indications. Pharmacol Rev 56:351–369
Thacker MA, Clark AK, Marchand F et al (2007) Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg 105:838–847
Truong W, Cheng C, Xu QG et al (2003) Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol 53:366–375
Uçeyler N, Sommer C (2008) Cytokine regulation in animal models of neuropathic pain and in human diseases. Neurosci Lett 437:194–198
Uçeyler N, Tscharke A, Sommer C (2007) Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun 21:553–560
Vougioukas VI, Roeske S, Michel U et al (1998) Wallerian degeneration in ICAM-1-deficient mice. Am J Pathol 152:241–249
Wagner R, Janjingian M, Myers RR (1998) Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain 74:35–42
Walczak JS, Pichette V, Leblond F et al (2005) Behavioral, pharmacological and molecular characterization of the saphenous nerve partial ligation: a new model of neuropathic pain. Neuroscience 132:1093–1102
Walczak JS, Pichette V, Leblond F et al (2006) Characterization of chronic constriction of the saphenous nerve, a model of neuropathic pain in mice showing rapid molecular and electrophysiological changes. J Neurosci Res 83:1310–1322
Wall PD, Devor M, Inbal R et al (1979) Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain 7:103–111
Watkins LR, Maier SF (2002) Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 82:981–1011
Wenk HN, Brederson JD, Honda CN (2006) Morphine directly inhibits nociceptors in inflamed skin. J Neurophysiol 95:2083–2097
Woolf CJ, Ma Q (2007) Nociceptors: noxious stimulus detectors. Neuron 55:353–364
Yaksh TL (1988) Substance P release from knee joint afferent terminals: modulation by opioids. Brain Res 458:319–324
Zak-Prelich M, McKenzie RC, Sysa-Jedrzejowska A et al (2003) Local immune responses and systemic cytokine responses in zoster: relationship to the development of postherpetic neuralgia. Clin Exp Immunol 131:318–323
Zelenka M, Schäfers M, Sommer C (2005) Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 116:257–263
Zhang X, Bao L, Shi TJ et al (1998) Down-regulation of mu-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience 82:223–240
Zuo Y, Perkins NM, Tracey DJ et al (2003) Inflammation and hyperalgesia induced by nerve injury in the rat: a key role of mast cells. Pain 105:467–479
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft, Klinische Forschergruppe 100 (MA 2437/1-4; HM).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Machelska, H. Dual Peripheral Actions of Immune Cells in Neuropathic Pain. Arch. Immunol. Ther. Exp. 59, 11–24 (2011). https://doi.org/10.1007/s00005-010-0106-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-010-0106-x