Abstract.
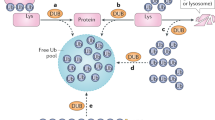
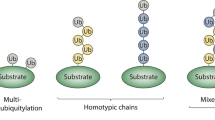
Ubiquitin is a highly conserved 76-aminoacid polypeptide that is found throughout the eukaryotic kingdom. The covalent conjugation of ubiquitin (often in the form of a polymer) to substrates governs a variety of biological processes ranging from proteolysis to DNA damage tolerance. The functional flexibility of this post-translational modification has its roots in the existence of a large number of ubiquitinating enzymes that catalyze the formation of distinct ubiquitin polymers, which in turn encode different signals. This review summarizes recent advances in the field with an emphasis on the non-canonical functions of polyubiquitination. We also discuss the potential mechanism of chain linkage specification as well as how structural disparity in ubiquitin polymers may be distinguished by ubiquitin receptors to translate the versatile ubiquitin signals into various cellular functions.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 20 February 2008; received after revision 12 March 2008; accepted 1 April 2008
Rights and permissions
About this article
Cite this article
Li, W., Ye, Y. Polyubiquitin chains: functions, structures, and mechanisms. Cell. Mol. Life Sci. 65, 2397–2406 (2008). https://doi.org/10.1007/s00018-008-8090-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-008-8090-6