Abstract
Several recent publications have described examples of physical and functional interations between tetraspanins and specific membrane proteases belonging to the TM-MMP and α-(ADAMs) and γ-secretases families. Collectively, these examples constitute an emerging body of evidence supporting the notion that tetraspanin-enriched microdomains (TEMs) represent functional platforms for the regulation of key cellular processes including the release of surface protein ectodomains ("shedding"), regulated intramembrane proteolysis ("RIPing") and matrix degradation and assembly. These cellular processes in turn play a crucial role in an array of physiological and pathological phenomena. Thus, TEMs may represent new therapeutical targets that may simultaneously affect the proteolytic activity of different enzymes and their substrates. Agonistic or antagonistic antibodies and blocking soluble peptides corresponding to tetraspanin functional regions may offer new opportunities in the treatment of pathologies such as chronic inflammation, cancer, or Alzheimer's disease. In this review article, we will discuss all these aspects of functional regulation of protease activities by tetraspanins.


Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer's disease
- ADAM:
-
A disintegrin and metalloprotease domain
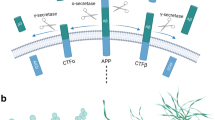
- APP:
-
Amyloid precursor protein
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- GPCRs:
-
G protein-coupled receptors
- HB-EGF:
-
Heparin-binding epidermal growth factor
- ICAM-1:
-
Intercellular adhesion molecule-1
- mAbs:
-
Monoclonal antibodies
- MT1-MMP:
-
Membrane-type-1-matrix metalloprotease
- PMA:
-
Phorbol-12-myristate-13-acetate
- RIP:
-
Regulated intramembrane proteolysis
- TEMs:
-
Tetraspanin-enriched microdomains
- TMPS:
-
Triple membrane-passing signaling
- TNFα:
-
Tumor necrosis factor-α
References
Charrin S, le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E (2009) Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J 420:133–154
Zoller M (2009) Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer 9:40–55
Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F (2009) Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 19:434–446
Hemler ME (2005) Tetraspanin functions and associated microdomains. Nature Rev 6:801–811
Edwards DR, Handsley MM, Pennington CJ (2008) The ADAM metalloproteinases. Mol Aspects Med 29:258–289
Blobel CP (2005) ADAMs: key components in EGFR signalling and development. Nature Rev 6:32–43
Reiss K, Saftig P (2009) The "a disintegrin and metalloprotease" (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol 20:126–137
Murphy G (2008) The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer 8:929–941
Pruessmeyer J, Ludwig A (2009) The good, the bad and the ugly substrates for ADAM10 and ADAM17 in brain pathology, inflammation and cancer. Semin Cell Dev Biol 20:164–174
Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC (2003) Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J 22:2704–2716
Sternlicht MD, Sunnarborg SW (2008) The ADAM17-amphiregulin-EGFR axis in mammary development and cancer. J Mammary Gland Biol Neoplasia 13:181–194
Fischer OM, Hart S, Gschwind A, Ullrich A (2003) EGFR signal transactivation in cancer cells. Biochem Soc Trans 31:1203–1208
Liebmann C (2010) EGF receptor activation by GPCRs: an universal pathway reveals different versions. Mol Cell Endocrinol 331:222–231
Ohtsu H, Dempsey PJ, Eguchi S (2006) ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol 291:C1–C10
Yan Y, Shirakabe K, Werb Z (2002) The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J Cell Biol 158:221–226
Horiuchi K, Le Gall S, Schulte M, Yamaguchi T, Reiss K, Murphy G, Toyama Y, Hartmann D, Saftig P, Blobel CP (2007) Substrate selectivity of epidermal growth factor-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Mol Biol Cell 18:176–188
Diaz-Rodriguez E, Montero JC, Esparis-Ogando A, Yuste L, Pandiella A (2002) Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol Biol Cell 13:2031–2044
Fan H, Turck CW, Derynck R (2003) Characterization of growth factor-induced serine phosphorylation of tumor necrosis factor-alpha converting enzyme and of an alternatively translated polypeptide. J Biol Chem 278:18617–18627
Soond SM, Everson B, Riches DW, Murphy G (2005) ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci 118:2371–2380
Nagano O, Murakami D, Hartmann D, De Strooper B, Saftig P, Iwatsubo T, Nakajima M, Shinohara M, Saya H (2004) Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol 165:893–902
Murphy G (2009) Regulation of the proteolytic disintegrin metalloproteinases, the ‘Sheddases’. Semin Cell Dev Biol 20:138–145
Arduise C, Abache T, Li L, Billard M, Chabanon A, Ludwig A, Mauduit P, Boucheix C, Rubinstein E, Le Naour F (2008) Tetraspanins regulate ADAM10-mediated cleavage of TNF-alpha and epidermal growth factor. J Immunol 181:7002–7013
Xu D, Sharma C, Hemler ME (2009) Tetraspanin12 regulates ADAM10-dependent cleavage of amyloid precursor protein. Faseb J 23:3674–3681
Gutiérrez-López M, Gilsanz A, Yánez-Mó M, Ovalle S, Lafuente M, Domínguez C, Monk P, González-Alvaro I, Sánchez-Madrid F, Cabañas C (2011) The sheddase activity of ADAM17/TACE is regulated by the tetraspanin CD9. Cell Mol Life Sci. doi:10.1007/s00018-011-0639-0
Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Gast D, Joumaa S, Zentgraf H, Fogel M, Altevogt DP (2003) ADAM10-mediated cleavage of L1 adhesion molecule at the cell surface and in released membrane vesicles. Faseb J 17:292–294
Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, Le Naour F, Gutwein P, Ludwig A, Rubinstein E, Altevogt P (2006) A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J 393:609–618
Keller S, Konig AK, Marme F, Runz S, Wolterink S, Koensgen D, Mustea A, Sehouli J, Altevogt P (2009) Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett 278:73–81
Soderberg A, Barral AM, Soderstrom M, Sander B, Rosen A (2007) Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic Biol Med 43:90–99
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593
Simons M, Raposo G (2009) Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–581
Zoller M (2006) Gastrointestinal tumors: metastasis and tetraspanins. Z Gastroenterol 44:573–586
Chen MS, Tung KS, Coonrod SA, Takahashi Y, Bigler D, Chang A, Yamashita Y, Kincade PW, Herr JC, White JM (1999) Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc Natl Acad Sci USA 96:11830–11835
Ziyyat A, Rubinstein E, Monier-Gavelle F, Barraud V, Kulski O, Prenant M, Boucheix C, Bomsel M, Wolf JP (2006) CD9 controls the formation of clusters that contain tetraspanins and the integrin alpha 6 beta 1, which are involved in human and mouse gamete fusion. J Cell Sci 119:416–424
Takahashi Y, Bigler D, Ito Y, White JM (2001) Sequence-specific interaction between the disintegrin domain of mouse ADAM 3 and murine eggs: role of beta1 integrin-associated proteins CD9, CD81, and CD98. Mol Biol Cell 12:809–820
Miller BJ, Georges-Labouesse E, Primakoff P, Myles DG (2000) Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J Cell Biol 149:1289–1296
Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C (2000) Severely reduced female fertility in CD9-deficient mice. Science 287:319–321
Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A (2000) The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet 24:279–282
Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E (2000) Requirement of CD9 on the egg plasma membrane for fertilization. Science 287:321–324
Rubinstein E, Ziyyat A, Prenant M, Wrobel E, Wolf JP, Levy S, Le Naour F, Boucheix C (2006) Reduced fertility of female mice lacking CD81. Dev Biol 290:351–358
Kaji K, Oda S, Miyazaki S, Kudo A (2002) Infertility of CD9-deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm-egg fusion. Dev Biol 247:327–334
Zhou GB, Liu GS, Meng QG, Liu Y, Hou YP, Wang XX, Li N, Zhu SE (2009) Tetraspanin CD9 in bovine oocytes and its role in fertilization. J Reprod Dev 55:305–308
Waterhouse R, Ha C, Dveksler GS (2002) Murine CD9 is the receptor for pregnancy-specific glycoprotein 17. J Exp Med 195:277–282
Ellerman DA, Ha C, Primakoff P, Myles DG, Dveksler GS (2003) Direct binding of the ligand PSG17 to CD9 requires a CD9 site essential for sperm–egg fusion. Mol Biol Cell 14:5098–5103
Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, Miyamoto K, Akutsu H, Kondo T, Takahashi Y, Ban T, Ito C, Toshimori K, Nakamura A, Ito M, Miyado M, Mekada E, Umezawa A (2008) The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc Natl Acad Sci USA 105:12921–12926
Gupta S, Primakoff P, Myles DG (2009) Can the presence of wild-type oocytes during insemination rescue the fusion defect of CD9 null oocytes? Mol Reprod Dev 76:602
Woo HN, Baik SH, Park JS, Gwon AR, Yang S, Yun YK, Jo DG (2011) Secretases as therapeutic targets for Alzheimer’s disease. Biochem Biophys Res Commun 404:10–15
Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Molecular cell 5:207–216
Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R (2000) A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Molecular cell 5:197–206
Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, Saftig P (2002) The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet 11:2615–2624
Srinivasan B, Wang Z, Brun-Zinkernagel AM, Collier RJ, Black RA, Frank SJ, Barker PA, Roque RS (2007) Photic injury promotes cleavage of p75NTR by TACE and nuclear trafficking of the p75 intracellular domain. Mol Cell Neurosci 36:449–461
Spasic D, Annaert W (2008) Building gamma-secretase: the bits and pieces. J Cell Sci 121:413–420
Wakabayashi T, Craessaerts K, Bammens L, Bentahir M, Borgions F, Herdewijn P, Staes A, Timmerman E, Vandekerckhove J, Rubinstein E, Boucheix C, Gevaert K, De Strooper B (2009) Analysis of the gamma-secretase interactome and validation of its association with tetraspanin-enriched microdomains. Nat Cell Biol 11:1340–1346
Ovalle S, Gutierrez-Lopez MD, Olmo N, Turnay J, Lizarbe MA, Majano P, Molina-Jimenez F, Lopez-Cabrera M, Yanez-Mo M, Sanchez-Madrid F, Cabanas C (2007) The tetraspanin CD9 inhibits the proliferation and tumorigenicity of human colon carcinoma cells. Int J Cancer 121:2140–2152
Sestan N, Artavanis-Tsakonas S, Rakic P (1999) Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science 286:741–746
Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D (2002) Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev 16:846–858
Poirazi P, Mel BW (2001) Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron 29:779–796
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376
Berezovska O, Xia MQ, Hyman BT (1998) Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J Neuropathol Exp Neurol 57:738–745
Dunn CD, Sulis ML, Ferrando AA, Greenwald I (2010) A conserved tetraspanin subfamily promotes Notch signaling in Caenorhabditis elegans and in human cells. Proc Natl Acad Sci USA 107:5907–5912
Hemler ME (2008) Targeting of tetraspanin proteins–potential benefits and strategies. Nat Rev Drug Discov 7:747–758
Lazo PA (2007) Functional implications of tetraspanin proteins in cancer biology. Cancer Sci 98:1666–1677
Cook GA, Wilkinson DA, Crossno J T Jr, Raghow R, Jennings LK (1999) The tetraspanin CD9 influences the adhesion, spreading, and pericellular fibronectin matrix assembly of Chinese hamster ovary cells on human plasma fibronectin. Exp Cell Res 251:356–371
Sachs N, Kreft M, h Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A (2006) Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175:33–39
Cowin AJ, Adams D, Geary SM, Wright MD, Jones JC, Ashman LK (2006) Wound healing is defective in mice lacking tetraspanin CD151. J Invest Dermatol 126:680–689
Sugiura T, Berditchevski F (1999) Function of alpha3beta1-tetraspanin protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2). J Cell Biol 146:1375–1389
Mazzocca A, Sciammetta SC, Carloni V, Cosmi L, Annunziato F, Harada T, Abrignani S, Pinzani M (2005) Binding of hepatitis C virus envelope protein E2 to CD81 up-regulates matrix metalloproteinase-2 in human hepatic stellate cells. J Biol Chem 280:11329–11339
Hong IK, Jin YJ, Byun HJ, Jeoung DI, Kim YM, Lee H (2006) Homophilic interactions of Tetraspanin CD151 up-regulate motility and matrix metalloproteinase-9 expression of human melanoma cells through adhesion-dependent c-Jun activation signaling pathways. J Biol Chem 281:24279–24292
Shi GM, Ke AW, Zhou J, Wang XY, Xu Y, Ding ZB, Devbhandari RP, Huang XY, Qiu SJ, Shi YH, Dai Z, Yang XR, Yang GH, Fan J (2010) CD151 modulates expression of matrix metalloproteinase 9 and promotes neoangiogenesis and progression of hepatocellular carcinoma. Hepatology (Baltimore, MD) 52:183–196
Hong IK, Kim YM, Jeoung DI, Kim KC, Lee H (2005) Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp Mol Med 37:230–239
Saito Y, Tachibana I, Takeda Y, Yamane H, He P, Suzuki M, Minami S, Kijima T, Yoshida M, Kumagai T, Osaki T, Kawase I (2006) Absence of CD9 enhances adhesion-dependent morphologic differentiation, survival, and matrix metalloproteinase-2 production in small cell lung cancer cells. Cancer Res 66:9557–9565
Liu WM, Cao YJ, Yang YJ, Li J, Hu Z, Duan EK (2006) Tetraspanin CD9 regulates invasion during mouse embryo implantation. J Mol Endocrinol 36:121–130
Tohami T, Drucker L, Shapiro H, Radnay J, Lishner M (2007) Overexpression of tetraspanins affects multiple myeloma cell survival and invasive potential. FASEB J 21:691–699
Shiomi T, Inoki I, Kataoka F, Ohtsuka T, Hashimoto G, Nemori R, Okada Y (2005) Pericellular activation of proMMP-7 (promatrilysin-1) through interaction with CD151. Lab Investig J Tech Methods Pathol 85:1489–1506
Jung K, Liu X, Chirco R, Fridman R, Kim H (2006) Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J 25:3934–3942
Lafleur MA, Xu D, Hemler ME (2009) Tetraspanin proteins regulate membrane type-1 matrix metalloproteinase-dependent pericellular proteolysis. Mol Biol Cell 20:2030–2040
Takino T, Miyamori H, Kawaguchi N, Uekita T, Seiki M, Sato H (2003) Tetraspanin CD63 promotes targeting and lysosomal proteolysis of membrane-type 1 matrix metalloproteinase. Biochem Biophys Res Commun 304:160–166
Yanez-Mo M, Barreiro O, Gonzalo P, Batista A, Megias D, Genis L, Sachs N, Sala-Valdes M, Alonso MA, Montoya MC, Sonnenberg A, Arroyo AG, Sanchez-Madrid F (2008) MT1-MMP collagenolytic activity is regulated through association with tetraspanin CD151 in primary endothelial cells. Blood 112:3217–3226
Sato H, Takino T (2010) Coordinate action of membrane-type matrix metalloproteinase-1 (MT1-MMP) and MMP-2 enhances pericellular proteolysis and invasion. Cancer Sci 101:843–847
Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H (1999) MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99:81–92
Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S (1998) Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 58:1048–1051
Oh J, Takahashi R, Adachi E, Kondo S, Kuratomi S, Noma A, Alexander DB, Motoda H, Okada A, Seiki M, Itoh T, Itohara S, Takahashi C, Noda M (2004) Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene 23:5041–5048
Galvez BG, Matias-Roman S, Yanez-Mo M, Vicente-Manzanares M, Sanchez-Madrid F, Arroyo AG (2004) Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell 15:678–687
Gingras D, Beliveau R (2010) Emerging concepts in the regulation of membrane-type 1 matrix metalloproteinase activity. Biochim Biophys Acta 1803:142–150
Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC (2007) MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J 26:1499–1510
Kolesnikova TV, Kazarov AR, Lemieux ME, Lafleur MA, Kesari S, Kung AL, Hemler ME (2009) Glioblastoma inhibition by cell surface immunoglobulin protein EWI-2, in vitro and in vivo. Neoplasia 11:77–86
Bass R, Werner F, Odintsova E, Sugiura T, Berditchevski F, Ellis V (2005) Regulation of urokinase receptor proteolytic function by the tetraspanin CD82. J Biol Chem 280:14811–14818
Kallquist L, Hansson M, Persson AM, Janssen H, Calafat J, Tapper H, Olsson I (2008) The tetraspanin CD63 is involved in granule targeting of neutrophil elastase. Blood 112:3444–3454
Le Naour F, Andre M, Boucheix C, Rubinstein E (2006) Membrane microdomains and proteomics: lessons from tetraspanin microdomains and comparison with lipid rafts. Proteomics 6:6447–6454
Shi W, Fan H, Shum L, Derynck R (2000) The tetraspanin CD9 associates with transmembrane TGF-alpha and regulates TGF-alpha-induced EGF receptor activation and cell proliferation. J Cell Biol 148:591–602
Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, Klagsbrun M, Mekada E (1995) The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol 128:929–938
Barreiro O, Yanez-Mo M, Sala-Valdes M, Gutierrez-Lopez MD, Ovalle S, Higginbottom A, Monk PN, Cabanas C, Sanchez-Madrid F (2005) Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood 105:2852–2861
Garton KJ, Gough PJ, Raines EW (2006) Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J Leukoc Biol 79:1105–1116
Tsakadze NL, Sithu SD, Sen U, English WR, Murphy G, D’Souza SE (2006) Tumor necrosis factor-alpha-converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1). J Biol Chem 281:3157–3164
Gearing AJ, Newman W (1993) Circulating adhesion molecules in disease. Immunol Today 14:506–512
van Kilsdonk JW, van Kempen LC, van Muijen GN, Ruiter DJ, Swart GW (2010) Soluble adhesion molecules in human cancers: sources and fates. Eur J Cell Biol 89:415–427
Barreiro O, Zamai M, Yanez-Mo M, Tejera E, Lopez-Romero P, Monk PN, Gratton E, Caiolfa VR, Sanchez-Madrid F (2008) Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol 183:527–542
Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, Tojo H, Yana I, Seiki M (2002) CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J 21:3949–3959
Marrero-Diaz R, Bravo-Cordero JJ, Megias D, Garcia MA, Bartolome RA, Teixido J, Montoya MC (2009) Polarized MT1-MMP-CD44 interaction and CD44 cleavage during cell retraction reveal an essential role for MT1-MMP in CD44-mediated invasion. Cell Motil Cytoskeleton 66:48–61
Stipp CS (2010) Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev Mol Med 12:e3
Dijkstra S, Kooij G, Verbeek R, van der Pol SM, Amor S, Geisert E E Jr, Dijkstra CD, van Noort JM, Vries HE (2008) Targeting the tetraspanin CD81 blocks monocyte transmigration and ameliorates EAE. Neurobiol Dis 31:413–421
Nakamoto T, Murayama Y, Oritani K, Boucheix C, Rubinstein E, Nishida M, Katsube F, Watabe K, Kiso S, Tsutsui S, Tamura S, Shinomura Y, Hayashi N (2009) A novel therapeutic strategy with anti-CD9 antibody in gastric cancers. J Gastroenterol 44:889–896
Robak T, Robak P, Smolewski P (2009) TRU-016, a humanized anti-CD37 IgG fusion protein for the potential treatment of B-cell malignancies. Curr Opin Investig Drugs 10:1383–1390
Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, Flamez P, Dequenne A, Godaux E, van Leuven F, Fahrenholz F (2004) A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest 113:1456–1464
Tachida Y, Nakagawa K, Saito T, Saido TC, Honda T, Saito Y, Murayama S, Endo T, Sakaguchi G, Kato A, Kitazume S, Hashimoto Y (2008) Interleukin-1 beta up-regulates TACE to enhance alpha-cleavage of APP in neurons: resulting decrease in Abeta production. J Neurochem 104:1387–1393
Kim ML, Zhang B, Mills IP, Milla ME, Brunden KR, Lee VM (2008) Effects of TNFalpha-converting enzyme inhibition on amyloid beta production and APP processing in vitro and in vivo. J Neurosci 28:12052–12061
Chow VW, Mattson MP, Wong PC, Gleichmann M (2009) An overview of APP processing enzymes and products. Neuromolecular Med 12:1–12
Woo HN, Park JS, Gwon AR, Arumugam TV, Jo DG (2009) Alzheimer’s disease and Notch signaling. Biochem Biophys Res Commun 390:1093–1097
Moss ML, Stoeck A, Yan W, Dempsey PJ (2008) ADAM10 as a target for anti-cancer therapy. Curr Pharm Biotechnol 9:2–8
Arribas J, Bech-Serra JJ, Santiago-Josefat B (2006) ADAMs, cell migration and cancer. Cancer Metastasis Rev 25:57–68
Klein-Soyer C, Azorsa DO, Cazenave JP, Lanza F (2000) CD9 participates in endothelial cell migration during in vitro wound repair. Arterioscler Thromb Vasc Biol 20:360–369
Sincock PM, Fitter S, Parton RG, Berndt MC, Gamble JR, Ashman LK (1999) PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J Cell Sci 112:833–844
Deissler H, Kuhn EM, Lang GE, Deissler H (2007) Tetraspanin CD9 is involved in the migration of retinal microvascular endothelial cells. Int J Mol Med 20:643–652
Zhang XA, Kazarov AR, Yang X, Bontrager AL, Stipp CS, Hemler ME (2002) Function of the tetraspanin CD151-alpha6beta1 integrin complex during cellular morphogenesis. Mol Biol Cell 13:1–11
Yanez-Mo M, Alfranca A, Cabanas C, Marazuela M, Tejedor R, Ursa MA, Ashman LK, de Landazuri MO, Sanchez-Madrid F (1998) Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with alpha3 beta1 integrin localized at endothelial lateral junctions. J Cell Biol 141:791–804
Dominguez-Jimenez C, Yanez-Mo M, Carreira A, Tejedor R, Gonzalez-Amaro R, Alvarez V, Sanchez-Madrid F (2001) Involvement of alpha3 integrin/tetraspanin complexes in the angiogenic response induced by angiotensin II. FASEB J 15:1457–1459
Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye W (2009) TSPAN12 regulates retinal vascular development by promoting Norrin-but not Wnt-induced FZD4/beta-catenin signaling. Cell 139:299–311
Takeda Y, Kazarov AR, Butterfield CE, Hopkins BD, Benjamin LE, Kaipainen A, Hemler ME (2007) Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood 109:1524–1532
Zuo H, Liu Z, Liu X, Yang J, Liu T, Wen S, Zhang XA, Cianflone K, Wang D (2009) CD151 gene delivery after myocardial infarction promotes functional neovascularization and activates FAK signaling. Molecular Med (Cambridge, Mass) 15:307–315
Acknowledgments
This work was supported by grants BFU2007-66443/BMC and BFU2010-19144/BMC from Ministerio de Ciencia e Innovación, a grant from Fundación de Investigación Médica Mutua Madrileña and by the RETICS Program RD08/0075-RIER (Red de Inflamación y Enfermedades Reumáticas) from Instituto de Salud Carlos III (to C.C.), a grant from Fundación de Investigación Médica Mutua Madrileña (to M.D.G.L.), and the grant PI080794 from Instituto de Salud Carlos III (to M.Y-M).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yáñez-Mó, M., Gutiérrez-López, M.D. & Cabañas, C. Functional interplay between tetraspanins and proteases. Cell. Mol. Life Sci. 68, 3323–3335 (2011). https://doi.org/10.1007/s00018-011-0746-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0746-y