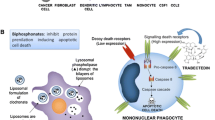
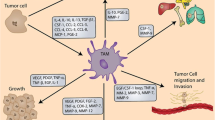
Abstract
Ample clinical and preclinical evidence indicates that macrophages interact with tumor cells as well as with virtually all populations of host cells present in the tumor microenvironment. This crosstalk can strongly promote malignancy, but also has in principle the potential to inhibit tumor growth. Thus, it is of the utmost importance to improve our understanding of the mechanisms driving the pro- and antimalignant behavior of tumor-associated macrophages (TAMs) in order to develop better anticancer therapies. In this review, we discuss the biological consequences of reciprocal interactions between TAMs, cancer cells, endothelial cells, fibroblasts and other leukocyte subfractions within tumors. It was recently elucidated that tumors specifically educate macrophages to secrete growth arrest-specific gene 6 (Gas6), the common ligand of the Tyro3, Axl, Mer receptor (TAMR) family. In turn, Gas6 fosters tumor growth by promoting cancer cell proliferation. Therefore, the Gas6–TAMR axis might represent a novel target for disrupting tumor–macrophage crosstalk. We summarize here what is known about TAMR and their ligands in (human) cancer biology. In order to shed more light on the role of macrophages in human cancer, we additionally provide an overview of what is currently known about the prognostic impact of TAMs in human cancer.



Similar content being viewed by others
References
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70 S0092-8674(00)81683-9[pii]
Allen M, Louise Jones J (2011) Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol 223(2):162–176. doi:10.1002/path.2803
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239–252. doi:10.1038/nrc2618
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. doi:10.1038/nature07205
Tlsty TD, Coussens LM (2006) Tumor stroma and regulation of cancer development. Annu Rev Pathol 1:119–150. doi:10.1146/annurev.pathol.1.110304.100224
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140(6):883–899. doi:10.1016/j.cell.2010.01.025
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1):39–51. doi:10.1016/j.cell.2010.03.014
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7(3):211–217. doi:10.1016/j.ccr.2005.02.013
DeClerck YA, Mercurio AM, Stack MS, Chapman HA, Zutter MM, Muschel RJ, Raz A, Matrisian LM, Sloane BF, Noel A, Hendrix MJ, Coussens L, Padarathsingh M (2004) Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol 164(4):1131–1139. doi:10.1016/S0002-9440(10)63200-2
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307. doi:10.1038/nature10144
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358(19):2039–2049. doi:10.1056/NEJMra0706596
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM (2009) Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16(3):183–194. doi:10.1016/j.ccr.2009.06.017
Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131(3):463–475
Shiai F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+ Gr1+ myeloid cells. Nat Biotechnol 25(8):911–920. doi:10.1038/nbt1323
Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17(3):320–329. doi:10.1038/nm.2328
Tiwari M (2010) From tumor immunology to cancer immunotherapy: miles to go. J Cancer Res Ther 6(4):427–431. doi:10.4103/0973-1482.77071
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30(7):1073–1081. doi:10.1093/carcin/bgp127
de Visser KE, Eichten A, Coussens LM (2006) Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 6(1):24–37. doi:10.1038/nrc1782
Mantovani A, Sica A (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 22(2):231–237. doi:10.1016/j.coi.2010.01.009
Pollard JW (2009) Trophic macrophages in development and disease. Nat Rev Immunol 9(4):259–270. doi:10.1038/nri2528
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124(2):263–266. doi:10.1016/j.cell.2006.01.007
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11(10):889–896. doi:10.1038/ni.1937
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5(12):953–964. doi:10.1038/nri1733
Hussain SP, Hofseth LJ, Harris CC (2003) Radical causes of cancer. Nat Rev Cancer 3(4):276–285. doi:10.1038/nrc1046
Jeannin P, Duluc D, Delneste Y (2011) IL-6 and leukemia-inhibitory factor are involved in the generation of tumor-associated macrophage: regulation by IFN-gamma. Immunotherapy 3(4 Suppl):23–26. doi:10.2217/imt.11.30
Heusinkveld M, van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, van Hall T, van der Burg SH (2011) M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol 187(3):1157–1165. doi:10.4049/jimmunol.1100889
Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE (2009) Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood 113(14):3139–3146. doi:10.1182/blood-2008-12-172825
Sica A, Bronte V (2007) Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 117(5):1155–1166. doi:10.1172/JCI31422
Jiang H, Harris MB, Rothman P (2000) IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol 105(6 Pt 1):1063–1070 S0091674900452255[pii]
Donnelly RP, Dickensheets H, Finbloom DS (1999) The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res 19(6):563–573. doi:10.1089/107999099313695
Martinez FO, Gordon S, Locati M, Mantovani A (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177(10):7303–7311 177/10/7303[pii]
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969. doi:10.1038/nri2448
Pettersen JS, Fuentes-Duculan J, Suarez-Farinas M, Pierson KC, Pitts-Kiefer A, Fan L, Belkin DA, Wang CQ, Bhuvanendran S, Johnson-Huang LM, Bluth MJ, Krueger JG, Lowes MA, Carucci JA (2011) Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J Invest Dermatol 131(6):1322–1330. doi:10.103/jid.2011.9
Rigo A, Gottardi M, Zamo A, Mauri P, Bonifacio M, Krampera M, Damiani E, Pizzolo G, Vinante F (2010) Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Mol Cancer 9:273. doi:10.1186/1476-4598-9-273
Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70(14):5728–5739. doi:10.1158/0008-5472.CAN-09-4672
Hamilton JA (2008) Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8(7):533–544. doi:10.1038/nri2356
Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B (2006) Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J Exp Clin Cancer Res 25(3):365–372
Lee CH, Espinosa I, Vrijaldenhoven S, Subramanian S, Montgomery KD, Zhu S, Marinelli RJ, Peterse JL, Poulin N, Nielsen TO, West RB, Gilks CB, van de Rijn M (2008) Prognostic significance of macrophage infiltration in leiomyosarcomas. Clin Cancer Res 14(5):1423–1430. doi:10.1158/1078-0432.CCR-07-1712
Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, Ohno Y, Suzuki N, Inoue M, Soma G, Nagasue N (2005) Role of tumor-associated macrophages (TAM) in advanced gastric carcinoma: the impact on FasL-mediated counterattack. Anticancer Res 25(1B):463–470
Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, Suzuki N, Inoue M, Soma G, Nagasue N (2003) The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res 23(6D):5015–5022
Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, Sjoegren P, Christensen IJ, Steiniche T (2009) Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol 27(20):3330–3337. doi:10.1200/JCO.2008.19.9919
Soeda S, Nakamura N, Ozeki T, Nishiyama H, Hojo H, Yamada H, Abe M, Sato A (2008) Tumor-associated macrophages correlate with vascular space invasion and myometrial invasion in endometrial carcinoma. Gynecol Oncol 109(1):122–128. doi:10.1016/j.ygyno.2007.12.033
Hsu HP, Shan YS, Lai MD, Lin PW (2010) Osteopontin-positive infiltrating tumor-associated macrophages in bulky ampullary cancer predict survival. Cancer Biol Ther 10(2):144–154
Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Terao T (2002) Thymidine phosphorylase expression in tumor-infiltrating macrophages may be correlated with poor prognosis in uterine endometrial cancer. Hum Pathol 33(11):1105–1113. doi:10.1053/hupa.2002.129203S0046817702001636
Wang R, Lu M, Zhang J, Chen S, Luo X, Qin Y, Chen H (2011) Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res 30:62. doi:10.1186/1756-9966-30-62
Bronkhorst IH, Ly LV, Jordanova ES, Vrolijk J, Versluis M, Luyten GP, Jager MJ (2011) Detection of M2-macrophages in uveal melanoma and relation with survival. Invest Ophthalmol Vis Sci 52(2):643–650. doi:10.1167/iovs.10-5979
Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, Beppu T, Baba H, Takeya M (2010) Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci 101(8):1913–1919. doi:10.1111/j.1349-7006.2010.01614.x
Ma J, Liu L, Che G, Yu N, Dai F, You Z (2010) The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 10:112. doi:10.1186/1471-2407-10-112
Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P (2009) Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 33(1):118–126. doi:10.1183/09031936.00065708
Markosyan N, Chen E, Ndong V, Yao Y, Sterner CJ, Chodosh LA, Lawson JA, Fitzgerald GA, Smyth EM (2011) Deletion of cyclooxygenase 2 in mouse mammary epithelial cells delays breast cancer onset through augmentation of type 1 immune responses in tumors. Carcinogenesis 32(10):1441–1449. doi:10.1093/carcin/bgr134
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Akerud P, De Mol M, Salomaki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P (2011) HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19(1):31–44. doi:10.1016/j.ccr.2010.11.009
Sanchez-Martin L, Estecha A, Samaniego R, Sanchez-Ramon S, Vega MA, Sanchez-Mateos P (2011) The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 117(1):88–97. doi:10.1182/blood-2009-12-258186
Deng L, Zhou JF, Sellers RS, Li JF, Nguyen AV, Wang Y, Orlofsky A, Liu Q, Hume DA, Pollard JW, Augenlicht L, Lin EY (2010) A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol 176(2):952–967. doi:10.2353/ajpath.2010.090622
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5(10):749–759. doi:10.1038/nri1703
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M (2004) IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118(3):285–296. doi:10.1016/j.cell.2004.07.013S0092867404006713
Maeda S, Kamata H, Luo JL, Leffert H, Karin M (2005) IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121(7):977–990. doi:10.1016/j.cell.2005.04.014
Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y (2004) NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431(7007):461–466. doi:10.1038/nature02924
Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR (2004) Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene 23(10):1902–1910. doi:10.1038/sj.onc.12073171207317
Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F (1999) Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med 5(7):828–831. doi:10.1038/10552
Balkwill F (2009) Tumour necrosis factor and cancer. Nat Rev Cancer 9(5):361–371. doi:10.1038/nrc2628
Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M (2008) Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J 27(12):1671–1681. doi:10.1038/emboj.2008.105
Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N (2008) Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118(2):560–570. doi:10.1172/JCI32453
Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR (2009) gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15(2):91–102. doi:10.1016/j.ccr.2009.01.002
Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7(1):41–51. doi:10.1038/nri1995
Karin M, Lawrence T, Nizet V (2006) Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124(4):823–835. doi:10.1016/j.cell.2006.02.016
Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317(5834):121–124. doi:10.1126/science.1140485
Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, Li M, Luo C, Yu S, Bian X, Feng H (2011) Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol 232(1–2):75–82. doi:10.1016/j.jneuroim.2010.10.011
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555 S1471490602023025[pii]
Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A (2006) A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 107(5):2112–2122. doi:10.1182/blood-2005-01-0428
Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, Mantovani A, Sica A (2009) Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A 106(35):14978–14983. doi:10.1073/pnas.0809784106
Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A (2006) p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res 66(23):11432–11440. doi:10.1158/0008-5472.CAN-06-1867
Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A (2000) Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol 164(2):762–767 ji_v164n2p762[pii]
Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA (2010) IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 24(3):241–255. doi:10.1101/gad.1874010
Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T (2009) M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 206(5):1089–1102. doi:10.1084/jem.20081605
Lin EY, Nguyen AV, Russell RG, Pollard JW (2001) Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193(6):727–740
Abraham D, Zins K, Sioud M, Lucas T, Schafer R, Stanley ER, Aharinejad S (2010) Stromal cell-derived CSF-1 blockade prolongs xenograft survival of CSF-1-negative neuroblastoma. Int J Cancer 126(6):1339–1352. doi:10.1002/ijc.24859
Aharinejad S, Sioud M, Lucas T, Abraham D (2009) Targeting stromal-cancer cell interactions with siRNAs. Methods Mol Biol 487:243–266
Loges S, Schmidt T, Carmeliet P (2009) “Antimyeloangiogenic” therapy for cancer by inhibiting PlGF. Clin Cancer Res 15(11):3648–3653. doi:10.1158/1078-0432.CCR-08-2276
Van de Veire S, Stalmans I, Heindryckx F, Oura H, Tijeras-Raballand A, Schmidt T, Loges S, Albrecht I, Jonckx B, Vinckier S, Van Steenkiste C, Tugues S, Rolny C, De Mol M, Dettori D, Hainaud P, Coenegrachts L, Contreres JO, Van Bergen T, Cuervo H, Xiao WH, Le Henaff C, Buysschaert I, Kharabi Masouleh B, Geerts A, Schomber T, Bonnin P, Lambert V, Haustraete J, Zacchigna S, Rakic JM, Jimenez W, Noel A, Giacca M, Colle I, Foidart JM, Tobelem G, Morales-Ruiz M, Vilar J, Maxwell P, Vinores SA, Carmeliet G, Dewerchin M, Claesson-Welsh L, Dupuy E, Van Vlierberghe H, Christofori G, Mazzone M, Detmar M, Collen D, Carmeliet P (2010) Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell 141(1):178–190. doi:10.1016/j.cell.2010.02.039
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64(19):7022–7029. doi:10.1158/0008-5472.CAN-04-1449
Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67(6):2649–2656. doi:10.1158/0008-5472.CAN-06-1823
Green CE, Liu T, Montel V, Lester RD, Subramaniam S, Gonias SL, Klemke RL (2009) Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. PLoS One 4(8):e6713. doi:10.1371/journal.pone.0006713
DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM (2009) CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16(2):91–102. doi:10.1016/j.ccr.2009.06.018
Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, Lee S (2007) Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci U S A 104(32):13092–13097. doi:10.1073/pnas.0702387104
Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC (2010) Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 120(8):2699–2714. doi:10.1172/JCI39506
Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW (2010) Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol 184(2):702–712. doi:10.4049/jimmunol.0902360
Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, Di Serio C, Naldini L, De Palma M (2009) A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood 114(4):901–914. doi:10.1182/blood-2009-01-200931
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66(23):11238–11246. doi:10.1158/0008-5472.CAN-06-1278
Luo YP, Zhou H, Krueger J, Kaplan C, Liao D, Markowitz D, Liu C, Chen T, Chuang TH, Xiang R, Reisfeld RA (2010) The role of proto-oncogene Fra-1 in remodeling the tumor microenvironment in support of breast tumor cell invasion and progression. Oncogene 29(5):662–673. doi:10.1038/onc.2009.308
Takanami I, Takeuchi K, Kodaira S (1999) Tumor-associated macrophage infiltration in pulmonary adenocarcinoma: association with angiogenesis and poor prognosis. Oncology 57(2):138–142
Murri AM, Hilmy M, Bell J, Wilson C, McNicol AM, Lannigan A, Doughty JC, McMillan DC (2008) The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br J Cancer 99(7):1013–1019. doi:10.1038/sj.bjc.6604667
Fraisl P, Mazzone M, Schmidt T, Carmeliet P (2009) Regulation of angiogenesis by oxygen and metabolism. Dev Cell 16(2):167–179. doi:10.1016/j.devcel.2009.01.003
Murdoch C, Muthana M, Coffelt SB, Lewis CE (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8(8):618–631
Carmeliet P, Jain RK (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 10(6):417–427. doi:10.1038/nrd3455
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62
De Bock K, Cauwenberghs S, Carmeliet P (2011) Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev 21(1):73–79. doi:10.1016/j.gde.2010.10.008
Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G (2008) HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13(3):206–220. doi:10.1016/j.ccr.2008.01.034
De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8(3):211–226
Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M (2011) Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19(4):512–526. doi:10.1016/j.ccr.2011.02.005
Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116(5):829–840. doi:10.1182/blood-2009-12-257832
Schmidt T, Carmeliet P (2010) Blood-vessel formation: bridges that guide and unite. Nature 465(7299):697–699. doi:10.1038/465697a
Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM (2005) Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest 115(2):247–257. doi:10.1172/JCI22037
Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K, Cha HJ, Schwendener RA, Jang KY, Kim KS, Alitalo K, Koh GY (2008) Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res 68(4):1100–1109. doi:10.1158/0008-5472.CAN-07-2572
Moussai D, Mitsui H, Pettersen JS, Pierson KC, Shah KR, Suarez-Farinas M, Cardinale IR, Bluth MJ, Krueger JG, Carucci JA (2011) The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J Invest Dermatol 131(1):229–236. doi:10.1038/jid.2010.266
Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G (2009) Myeloid cells contribute to tumor lymphangiogenesis. PLoS One 4(9):e7067. doi:10.1371/journal.pone.0007067
Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, Gao JF (2010) Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med Oncol. doi:10.1007/s12032-010-9638-5
Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S (2011) Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res 167(2):e211–e219. doi:10.1016/j.jss.2009.05.026
Zhang J, Chen L, Xiao M, Wang C, Qin Z (2010) FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol 178(1):382–390. doi:10.1016/j.ajpath.2010.11.017
Erez N, Truitt M, Olson P, Arron ST, Hanahan D (2010) Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17(2):135–147. doi:10.1016/j.ccr.2009.12.041
Silzle T, Kreutz M, Dobler MA, Brockhoff G, Knuechel R, Kunz-Schughart LA (2003) Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur J Immunol 33(5):1311–1320. doi:10.1002/eji.200323057
Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA (2009) Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One 4(11):e7965. doi:10.1371/journal.pone.0007965
Rama D, Esendagli G, Guc D (2011) Expression of chemokine-like receptor 1 (CMKLR1) on J744A.1 macrophages co-cultured with fibroblast and/or tumor cells: modeling the influence of microenvironment. Cell Immunol 271(1):134–140. doi:10.1016/j.cellimm.2011.06.016
Sinha P, Clements VK, Ostrand-Rosenberg S (2005) Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res 65(24):11743–11751. doi:10.1158/0008-5472.CAN-05-0045
Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS (2010) Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 70(19):7465–7475. doi:10.1158/0008-5472.CAN-10-1439
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L (2009) Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 206(6):1327–1337. doi:10.1084/jem.20082173
Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L (2009) Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer 125(7):1640–1648. doi:10.1002/ijc.24556
Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, Wang H, Chen J (2011) Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One 6(4):e19495. doi:10.1371/journal.pone
Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, Zhang B, Liu T, Yang P (2011) Foxp3+ IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol 89(1):85–91. doi:10.1189/jlb.0910506
Galani IE, Wendel M, Stojanovic A, Jesiak M, Muller MM, Schellack C, Suri-Payer E, Cerwenka A (2010) Regulatory T cells control macrophage accumulation and activation in lymphoma. Int J Cancer 127(5):1131–1140. doi:10.1002/ijc.25132
Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV (2009) Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol 9:65. doi:10.1186/1471-230X-9-65
Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, Abastado JP, Lam KP, Biswas SK (2010) Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol 40(8):2296–2307. doi:10.1002/eji.200940288
DeNardo DG, Brennan DJ, Rexhapaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirström K, West BL, Coussens LM (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discovery 1(1):54–67
Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q (2009) Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 114(17):3625–3628. doi:10.1182/blood-2009-05-220285
Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H (2011) Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A 108(30):12425–12430. doi:10.1073/pnas.1106645108
Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld MG, Rose DW (2006) Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell 124(3):615–629. doi:10.1016/j.cell.2005.12.032
Shiai F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, Ho C, Ross J, Tan M, Carano RA, Meng YG, Ferrara N (2007) Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450(7171):825–831. doi:10.1038/nature06348
Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML, Wu L (2011) Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 115(7):1461–1471. doi:10.1182/blood-2009-08-237412
Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, Adinin R, Overman MJ, Valero V, Wen S, Lieu C, Yan S, Tran HT, Ellis LM, Abbruzzese JL, Heymach JV (2010) Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 28(3):453–459. doi:10.1200/JCO.2009.24.8252
Loges S, Schmidt T, Carmeliet P (2010) Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer 1(1):12–25. doi:10.1177/1947601909356574
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141(7):1117–1134. doi:10.1016/j.cell.2010.06.011
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103(2):211–225 S0092-8674(00)00114-8[pii]
Lapraz F, Rottinger E, Duboc V, Range R, Duloquin L, Walton K, Wu SY, Bradham C, Loza MA, Hibino T, Wilson K, Poustka A, McClay D, Angerer L, Gache C, Lepage T (2006) RTK and TGF-beta signaling pathways genes in the sea urchin genome. Dev Biol 300(1):132–152. doi:10.1016/j.ydbio.2006.08.048
Satou Y, Imai KS, Levine M, Kohara Y, Rokhsar D, Satoh N (2003) A genomewide survey of developmentally relevant genes in Ciona intestinalis. I. Genes for bHLH transcription factors. Dev Genes Evol 213(5-6):213–221. doi:10.1007/s00427-003-0319-7
Lai C, Lemke G (1991) An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron 6(5):691–704 0896-6273(91)90167-X[pii]
O’Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R 3rd, Le Beau MM, Earp HS, Liu ET (1991) axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol 11(10):5016–5031
Sasaki T, Knyazev PG, Clout NJ, Cheburkin Y, Gohring W, Ullrich A, Timpl R, Hohenester E (2006) Structural basis for Gas6-Axl signalling. EMBO J 25(1):80–87. doi:10.1038/sj.emboj.7600912
Lemke G, Rothlin CV (2008) Immunobiology of the TAM receptors. Nat Rev Immunol 8(5):327–336. doi:10.1038/nri2303
Hafizi S, Dahlback B (2006) Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J 273(23):5231–5244. doi:10.1111/j.1742-4658.2006.05529.x
Linger RM, Keating AK, Earp HS, Graham DK (2011) Taking aim at Mer and Axl receptor tyrosine kinases as novel therapeutic targets in solid tumors. Expert Opin Ther Targets 14(10):1073–1090. doi:10.1517/14728222.2010.515980
Zhang QK, Boast S, de los Santos K, Begemann M, Goff SP (1996) Transforming activity of retroviral genomes encoding Gag-Axl fusion proteins. J Virol 70(11):8089–8097
Faust M, Ebensperger C, Schulz AS, Schleithoff L, Hameister H, Bartram CR, Janssen JW (1992) The murine ufo receptor: molecular cloning, chromosomal localization and in situ expression analysis. Oncogene 7(7):1287–1293
Angelillo-Scherrer A, Burnier L, Flores N, Savi P, DeMol M, Schaeffer P, Herbert JM, Lemke G, Goff SP, Matsushima GK, Earp HS, Vesin C, Hoylaerts MF, Plaisance S, Collen D, Conway EM, Wehrle-Haller B, Carmeliet P (2005) Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J Clin Invest 115(2):237–246. doi:10.1172/JCI22079
Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, Dewerchin M, Hoylaerts M, Herbert J, Collen D, Dahlback B, Carmeliet P (2001) Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med 7(2):215–221. doi:10.1038/84667
Linger RM, Keating AK, Earp HS, Graham DK (2008) TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 100:35–83. doi:10.1016/S0065-230X(08)00002-X
Bellosta P, Zhang Q, Goff SP, Basilico C (1997) Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene 15(20):2387–2397. doi:10.1038/sj.onc.1201419
Bellosta P, Costa M, Lin DA, Basilico C (1995) The receptor tyrosine kinase ARK mediates cell aggregation by homophilic binding. Mol Cell Biol 15(2):614–625
Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M (2001) Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer 37(17):2264–2274 S0959-8049(01)00271-4[pii]
Neubauer A, Burchert A, Maiwald C, Gruss HJ, Serke S, Huhn D, Wittig B, Liu E (1997) Recent progress on the role of Axl, a receptor tyrosine kinase, in malignant transformation of myeloid leukemias. Leuk Lymphoma 25(1–2):91–96. doi:10.3109/10428199709042499
Ou WB, Corson JM, Flynn DL, Lu WP, Wise SC, Bueno R, Sugarbaker DJ, Fletcher JA (2011) AXL regulates mesothelioma proliferation and invasiveness. Oncogene 30(14):1643–1652. doi:10.1038/onc.2010.555
Avilla E, Guarino V, Visciano C, Liotti F, Svelto M, Krishnamoorthy G, Franco R, Melillo RM (2011) Activation of TYRO3/AXL tyrosine kinase receptors in thyroid cancer. Cancer Res 71(5):1792–1804. doi:10.1158/0008-5472.CAN-10-2186
Pierce A, Bliesner B, Xu M, Nielsen-Preiss S, Lemke G, Tobet S, Wierman ME (2008) Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration. Mol Endocrinol 22(11):2481–2495. doi:10.1210/me.2008-0169
Scutera S, Fraone T, Musso T, Cappello P, Rossi S, Pierobon D, Orinska Z, Paus R, Bulfone-Paus S, Giovarelli M (2009) Survival and migration of human dendritic cells are regulated by an IFN-alpha-inducible Axl/Gas6 pathway. J Immunol 183(5):3004–3013. doi:10.4049/jimmunol.0804384
Song X, Wang H, Logsdon CD, Rashid A, Fleming JB, Abbruzzese JL, Gomez HF, Evans DB (2011) Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer 117(4):734–743. doi:10.1002/cncr.25483
Keating AK, Kim GK, Jones AE, Donson AM, Ware K, Mulcahy JM, Salzberg DB, Foreman NK, Liang X, Thorburn A, Graham DK (2010) Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther 9(5):1298–1307. doi:10.1158/1535-7163.MCT-09-0707
Papadakis ES, Cichon MA, Vyas JJ, Patel N, Ghali L, Cerio R, Storey A, O’Toole EA (2010) Axl promotes cutaneous squamous cell carcinoma survival through negative regulation of pro-apoptotic Bcl-2 family members. J Invest Dermatol 131(2):509–517. doi:10.1038/jid.2010.326
Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, Weimer R, Wu Y, Pei L (2010) An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 29(38):5254–5264
Polvi A, Armstrong E, Lai C, Lemke G, Huebner K, Spritz RA, Guida LC, Nicholls RD, Alitalo K (1993) The human TYRO3 gene and pseudogene are located in chromosome 15q14-q25. Gene 134(2):289–293
Ohashi K, Mizuno K, Kuma K, Miyata T, Nakamura T (1994) Cloning of the cDNA for a novel receptor tyrosine kinase, Sky, predominantly expressed in brain. Oncogene 9(3):699–705
Fujimoto J, Yamamoto T (1994) Brt, a mouse gene encoding a novel receptor-type protein-tyrosine kinase, is preferentially expressed in the brain. Oncogene 9(3):693–698
Crosier PS, Freeman SA, Orlic D, Bodine DM, Crosier KE (1996) The Dtk receptor tyrosine kinase, which binds protein S, is expressed during hematopoiesis. Exp Hematol 24(2):318–323
Jansa Perez M, Walshe JA, Crosier KE, Crosier PS (1996) Expression of the DTK receptor tyrosine kinase during zebrafish development. Int J Dev Biol Suppl 1:101S–102S
Biscardi JS, Denhez F, Buehler GF, Chesnutt DA, Baragona SC, O’Bryan JP, Der CJ, Fiordalisi JJ, Fults DW, Maness PF (1996) Rek, a gene expressed in retina and brain, encodes a receptor tyrosine kinase of the Axl/Tyro3 family. J Biol Chem 271(46):29049–29059
Mark MR, Scadden DT, Wang Z, Gu Q, Goddard A, Godowski PJ (1994) rse, a novel receptor-type tyrosine kinase with homology to Axl/Ufo, is expressed at high levels in the brain. J Biol Chem 269(14):10720–10728
Schulz NT, Paulhiac CI, Lee L, Zhou R (1995) Isolation and expression analysis of tyro3, a murine growth factor receptor tyrosine kinase preferentially expressed in adult brain. Brain Res Mol Brain Res 28(2):273–280 0169328X94002162[pii]
Healy AM, Schwartz JJ, Zhu X, Herrick BE, Varnum B, Farber HW (2001) Gas 6 promotes Axl-mediated survival in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 280(6):L1273–L1281
Nakamura YS, Hakeda Y, Takakura N, Kameda T, Hamaguchi I, Miyamoto T, Kakudo S, Nakano T, Kumegawa M, Suda T (1998) Tyro 3 receptor tyrosine kinase and its ligand, Gas6, stimulate the function of osteoclasts. Stem Cells 16(3):229–238. doi:10.1002/stem.160229
Dai W, Pan H, Hassanain H, Gupta SL, Murphy MJ Jr (1994) Molecular cloning of a novel receptor tyrosine kinase, tif, highly expressed in human ovary and testis. Oncogene 9(3):975–979
Wang H, Chen S, Chen Y, Wu H, Tang H, Xiong W, Ma J, Ge Y, Lu Q, Han D (2007) The role of Tyro 3 subfamily receptors in the regulation of hemostasis and megakaryocytopoiesis. Haematologica 92(5):643–650
Zhong Z, Wang Y, Guo H, Sagare A, Fernandez JA, Bell RD, Barrett TM, Griffin JH, Freeman RS, Zlokovic BV (2010) Protein S protects neurons from excitotoxic injury by activating the TAM receptor Tyro3-phosphatidylinositol 3-kinase-Akt pathway through its sex hormone-binding globulin-like region. J Neurosci 30(46):15521–15534. doi:10.1523/JNEUROSCI.4437-10.2010
Lan Z, Wu H, Li W, Wu S, Lu L, Xu M, Dai W (2000) Transforming activity of receptor tyrosine kinase tyro3 is mediated, at least in part, by the PI3 kinase-signaling pathway. Blood 95(2):633–638
Taylor IC, Roy S, Varmus HE (1995) Overexpression of the Sky receptor tyrosine kinase at the cell surface or in the cytoplasm results in ligand-independent activation. Oncogene 11(12):2619–2626
Zhu S, Wurdak H, Wang Y, Galkin A, Tao H, Li J, Lyssiotis CA, Yan F, Tu BP, Miraglia L, Walker J, Sun F, Orth A, Schultz PG, Wu X (2009) A genomic screen identifies TYRO3 as a MITF regulator in melanoma. Proc Natl Acad Sci U S A 106(40):17025–17030. doi:10.1073/pnas.0909292106
Heiring C, Dahlback B, Muller YA (2004) Ligand recognition and homophilic interactions in Tyro3: structural insights into the Axl/Tyro3 receptor tyrosine kinase family. J Biol Chem 279(8):6952–6958. doi:10.1074/jbc.M311750200
Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS (1994) Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ 5(6):647–657
Jia R, Hanafusa H (1994) The proto-oncogene of v-eyk (v-ryk) is a novel receptor-type protein tyrosine kinase with extracellular Ig/GN-III domains. J Biol Chem 269(3):1839–1844
Graham DK, Bowman GW, Dawson TL, Stanford WL, Earp HS, Snodgrass HR (1995) Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene 10(12):2349–2359
Chen C, Li Q, Darrow AL, Wang Y, Derian CK, Yang J, de Garavilla L, Andrade-Gordon P, Damiano BP (2004) Mer receptor tyrosine kinase signaling participates in platelet function. Arterioscler Thromb Vasc Biol 24(6):1118–1123. doi:10.1161/01.ATV.0000130662.30537.0801
Ling L, Kung HJ (1995) Mitogenic signals and transforming potential of Nyk, a newly identified neural cell adhesion molecule-related receptor tyrosine kinase. Mol Cell Biol 15(12):6582–6592
Lemke G, Lu Q (2003) Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol 15(1):31–36 S095279150200016X[pii]
Binder MD, Kilpatrick TJ (2009) TAM receptor signalling and demyelination. Neurosignals 17(4):277–287. doi:10.1159/000231894
Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA (2002) Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med 196(1):135–140
Rothlin CV, Lemke G (2010) TAM receptor signaling and autoimmune disease. Curr Opin Immunol 22(6):740–746. doi:10.1016/j.coi.2010.10.001
Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411(6834):207–211. doi:10.1038/35075603
Todt JC, Hu B, Curtis JL (2004) The receptor tyrosine kinase MerTK activates phospholipase C gamma2 during recognition of apoptotic thymocytes by murine macrophages. J Leukoc Biol 75(4):705–713. doi:10.1189/jlb.0903439
Ostergaard E, Duno M, Batbayli M, Vilhelmsen K, Rosenberg T (2011) A novel MERTK deletion is a common founder mutation in the Faroe Islands and is responsible for a high proportion of retinitis pigmentosa cases. Mol Vis 17:1485–1492
Wu YM, Robinson DR, Kung HJ (2004) Signal pathways in up-regulation of chemokines by tyrosine kinase MER/NYK in prostate cancer cells. Cancer Res 64(20):7311–7320. doi:10.1158/0008-5472.CAN-04-0972
Gyorffy B, Lage H (2007) A web-based data warehouse on gene expression in human malignant melanoma. J Invest Dermatol 127(2):394–399. doi:10.1038/sj.jid.5700543
Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF et al (1995) The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 80(4):661–670 0092-8674(95)90520-0[pii]
Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ et al (1995) Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature 373(6515):623–626. doi:10.1038/373623a0
Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K (1996) Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 271(47):30022–30027
Godowski PJ, Mark MR, Chen J, Sadick MD, Raab H, Hammonds RG (1995) Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell 82(3):355–358 0092-8674(95)90424-7[pii]
Mark MR, Chen J, Hammonds RG, Sadick M, Godowsk PJ (1996) Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J Biol Chem 271(16):9785–9789
Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, Lemke G (2006) TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci 33(1):96–108. doi:10.1016/j.mcn.2006.06.011
Schneider C, King RM, Philipson L (1988) Genes specifically expressed at growth arrest of mammalian cells. Cell 54(6):787–793 S0092-8674(88)91065-3[pii]
Dahlback B (1991) Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb Haemost 66(1):49–61
Manfioletti G, Brancolini C, Avanzi G, Schneider C (1993) The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol 13(8):4976–4985
Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK (2007) A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109(3):1026–1033. doi:10.1182/blood-2006-05-021634
Angelillo-Scherrer A, Burnier L, Lambrechts D, Fish RJ, Tjwa M, Plaisance S, Sugamele R, DeMol M, Martinez-Soria E, Maxwell PH, Lemke G, Goff SP, Matsushima GK, Earp HS, Chanson M, Collen D, Izui S, Schapira M, Conway EM, Carmeliet P (2008) Role of Gas6 in erythropoiesis and anemia in mice. J Clin Invest 118(2):583–596. doi:10.1172/JCI30375
Tjwa M, Bellido-Martin L, Lin Y, Lutgens E, Plaisance S, Bono F, Delesque-Touchard N, Herve C, Moura R, Billiau AD, Aparicio C, Levi M, Daemen M, Dewerchin M, Lupu F, Arnout J, Herbert JM, Waer M, Garcia de Frutos P, Dahlback B, Carmeliet P, Hoylaerts MF, Moons L (2008) Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes. Blood 111(8):4096–4105. doi:10.1182/blood-2007-05-089565
Lutgens E, Tjwa M, Garcia de Frutos P, Wijnands E, Beckers L, Dahlback B, Daemen MJ, Carmeliet P, Moons L (2008) Genetic loss of Gas6 induces plaque stability in experimental atherosclerosis. J Pathol 216(1):55–63. doi:10.1002/path.2381
Burnier L, Saller F, Kadi L, Brisset AC, Sugamele R, Baudino L, Bono F, Herbert JM, Carmeliet P, Schapira M, Izui S, Angelillo-Scherrer A (2011) Gas6 deficiency in recipient mice of allogeneic transplantation alleviates hepatic graft-versus-host disease. Blood 115(16):3390–3397. doi:10.1182/blood-2009-02-206920
Llacuna L, Barcena C, Bellido-Martin L, Fernandez L, Stefanovic M, Mari M, Garcia-Ruiz C, Fernandez-Checa JC, Garcia de Frutos P, Morales A (2010) Growth arrest-specific protein 6 is hepatoprotective against murine ischemia/reperfusion injury. Hepatology 52(4):1371–1379. doi:10.1002/hep.23833
Yin JL, Pilmore HL, Yan YQ, McCaughan GW, Bishop GA, Hambly BD, Eris JM (2002) Expression of growth arrest-specific gene 6 and its receptors in a rat model of chronic renal transplant rejection. Transplantation 73(4):657–660
Yin JL, Hambly BD, Bao SS, Painter D, Bishop GA, Eris JM (2003) Expression of growth arrest-specific gene 6 and its receptors in dysfunctional human renal allografts. Transpl Int 16(9):681–688. doi:10.1007/s00147-003-0593-3
Loges S, Schmidt T, Tjwa M, van Geyte K, Lievens D, Lutgens E, Vanhoutte D, Borgel D, Plaisance S, Hoylaerts M, Luttun A, Dewerchin M, Jonckx B, Carmeliet P (2010) Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood 115(11):2264–2273. doi:10.1182/blood-2009-06-228684
Goruppi S, Chiaruttini C, Ruaro ME, Varnum B, Schneider C (2001) Gas6 induces growth, beta-catenin stabilization, and T-cell factor transcriptional activation in contact-inhibited C57 mammary cells. Mol Cell Biol 21(3):902–915. doi:10.1128/MCB.21.3.902-915.2001
Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC (2005) Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol 204(1):36–44. doi:10.1002/jcp.20265
van Ginkel PR, Gee RL, Shearer RL, Subramanian L, Walker TM, Albert DM, Meisner LF, Varnum BC, Polans AS (2004) Expression of the receptor tyrosine kinase Axl promotes ocular melanoma cell survival. Cancer Res 64(1):128–134
Koessler W, Fiebeler A, Willms A, Elaidi T, Klosterhalfen B, Klinge U (2011) Formation of translational risk score based on correlation coefficients as an alternative to Cox regression models for predicting outcome in patients with NSCLC. Theor Biol Med Model 8(1):28. doi:10.1186/1742-4682-8-28
Smiley ST, Boyer SN, Heeb MJ, Griffin JH, Grusby MJ (1997) Protein S is inducible by interleukin 4 in T cells and inhibits lymphoid cell procoagulant activity. Proc Natl Acad Sci U S A 94(21):11484–11489
Caberoy NB, Zhou Y, Li W (2010) Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. EMBO J 29(23):3898–3910. doi:10.1038/emboj.2010.265
Li W (2011) Eat-me signals: keys to molecular phagocyte biology and “appetite” control. J Cell Physiol. doi:10.1002/jcp.22815
Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, Duan M, Torneros A, Yu J, Heckrodt TJ, Zhang J, Ding P, Apatira A, Chua J, Brandt R, Pine P, Goff D, Singh R, Payan DG, Hitoshi Y (2010) R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res 70(4):1544–1554. doi:10.1158/0008-5472.CAN-09-2997
Gustafsson A, Martuszewska D, Johansson M, Ekman C, Hafizi S, Ljungberg B, Dahlback B (2009) Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res 15(14):4742–4749. doi:10.1158/1078-0432.CCR-08-2514
Neubauer A, Fiebeler A, Graham DK, O’Bryan JP, Schmidt CA, Barckow P, Serke S, Siegert W, Snodgrass HR, Huhn D et al (1994) Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood 84(6):1931–1941
Challier C, Uphoff CC, Janssen JW, Drexler HG (1996) Differential expression of the ufo/axl oncogene in human leukemia-lymphoma cell lines. Leukemia 10(5):781–787
Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT, Lai GM, Chuang SE (2008) Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett 268(2):314–324. doi:10.1016/j.canlet.2008.04.017
Rochlitz C, Lohri A, Bacchi M, Schmidt M, Nagel S, Fopp M, Fey MF, Herrmann R, Neubauer A (1999) Axl expression is associated with adverse prognosis and with expression of Bcl-2 and CD34 in de novo acute myeloid leukemia (AML): results from a multicenter trial of the Swiss Group for Clinical Cancer Research (SAKK). Leukemia 13(9):1352–1358
Grosso S, Puissant A, Dufies M, Colosetti P, Jacquel A, Lebrigand K, Barbry P, Deckert M, Cassuto JP, Mari B, Auberger P (2009) Gene expression profiling of imatinib and PD166326-resistant CML cell lines identifies Fyn as a gene associated with resistance to BCR-ABL inhibitors. Mol Cancer Ther 8(7):1924–1933. doi:10.1158/1535-7163.MCT-09-0168
Gioia R, Leroy C, Drullion C, Lagarde V, Etienne G, Dulucq S, Lippert E, Roche S, Mahon FX, Pasquet JM (2011) Quantitative phosphoproteomics revealed interplay between Syk and Lyn in the resistance to nilotinib in chronic myeloid leukemia cells. Blood 118(8):2211–2221. doi:10.1182/blood-2010-10-313692
Ghosh AK, Secreto C, Boysen J, Sassoon T, Shanafelt TD, Mukhopadhyay D, Kay NE (2011) The novel receptor tyrosine kinase Axl is constitutively active in B-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: implications for therapy. Blood 117(6):1928–1937. doi:10.1182/blood-2010-09-305649
Graham DK, Salzberg DB, Kurtzberg J, Sather S, Matsushima GK, Keating AK, Liang X, Lovell MA, Williams SA, Dawson TL, Schell MJ, Anwar AA, Snodgrass HR, Earp HS (2006) Ectopic expression of the proto-oncogene Mer in pediatric T-cell acute lymphoblastic leukemia. Clin Cancer Res 12(9):2662–2669. doi:10.1158/1078-0432.CCR-05-2208
Linger RM, DeRyckere D, Brandao L, Sawczyn KK, Jacobsen KM, Liang X, Keating AK, Graham DK (2009) Mer receptor tyrosine kinase is a novel therapeutic target in pediatric B-cell acute lymphoblastic leukemia. Blood 114(13):2678–2687. doi:10.1182/blood-2009-03-209247
Shiozawa Y, Pedersen EA, Taichman RS (2010) GAS6/Mer axis regulates the homing and survival of the E2A/PBX1-positive B-cell precursor acute lymphoblastic leukemia in the bone marrow niche. Exp Hematol 38(2):132–140. doi:10.1016/j.exphem.2009.11.002
Dai W, Pan HQ, Ouyang B, Greenberg JM, Means RT Jr, Li B, Cardie J (1996) Expression of receptor protein tyrosine kinase tif is regulated during leukemia cell differentiation. Leukemia 10(6):978–983
Acknowledgments
S.L. is funded by the Max-Eder group leader program of the Deutsche Krebshilfe, by the Hamburger Krebsgesellschaft and by the Roggenbuck Stiftung. A.S. receives funding from the Roggenbuck Stiftung.
Author information
Authors and Affiliations
Corresponding author
Additional information
Thomas Schmidt and Isabel Ben-Batalla contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, T., Ben-Batalla, I., Schultze, A. et al. Macrophage–tumor crosstalk: role of TAMR tyrosine kinase receptors and of their ligands. Cell. Mol. Life Sci. 69, 1391–1414 (2012). https://doi.org/10.1007/s00018-011-0863-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0863-7