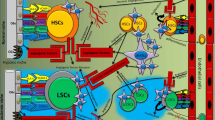
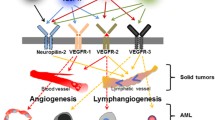
Abstract
This review is designed to provide an overview of the current literature concerning vascular endothelial growth factor signaling (VEGF) in acute myeloid leukemia (AML). Aberrant VEGF signaling operates in the bone marrow of AML patients and is related to a poor prognosis. The altered signaling pathway demonstrated to interfere in several autocrine and paracrine signaling pathways. VEGF signaling promotes autocrine AML blast cell proliferation, survival, and chemotherapy resistance. In addition, VEGF signaling can mediate paracrine vascular endothelial cell-controlled angiogenesis in AML. Both effects presumably explain the association of high VEGF levels and poor therapeutic outcome. More recently, researches focusing on bone marrow stem cell niches demonstrate a role for VEGF signaling in the preservation of several cell types within these niches. The bone marrow niches are proposed to be a protective microenvironment for AML cells that could be responsible for relapses in AML patients. This implies the need of sophisticated VEGF-targeted therapeutics in AML therapy strategies. This review highlights our current understanding of aberrant VEGF signaling in AML, appoints the interference of VEGF signaling in the AML-associated microenvironment, and reflects the novelty of current VEGF-targeted therapeutics used in clinical trails for the treatment of AML.




Similar content being viewed by others
References
Abrahamsson J, Forestier E, Heldrup J et al (2011) Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol 29:310–315
Faderl S, Ravandi F, Huang X et al (2012) Clofarabine plus low-dose cytarabine followed by clofarabine plus low-dose cytarabine alternating with decitabine in acute myeloid leukemia frontline therapy for older patients. Cancer. doi:10.1002
Gallegos-Castorena S, Medina-Sanson A, Gonzalez-Ramella O, Sanchez-Zubieta F, Martinez-Avalos A (2009) Improved treatment results in Mexican children with acute myeloid leukemia using a Medical Research Council (MRC)-acute myeloid leukemia 10 modified protocol. Leuk Lymphoma 50:1132–1137
Gerr H, Zimmermann M, Schrappe M et al (2010) Acute leukaemias of ambiguous lineage in children: characterization, prognosis and therapy recommendations. Br J Haematol 149:84–92
Hann IM, Webb DK, Gibson BE, Harrison CJ (2004) MRC trials in childhood acute myeloid leukaemia. Ann Hematol 83(Suppl 1):S108–S112
Derolf AR, Kristinsson SY, Andersson TM, Landgren O, Dickman PW, Bjorkholm M (2009) Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood 113:3666–3672
Grimwade D, Hills RK (2009) Independent prognostic factors for AML outcome. Hematology Am Soc Hematol Educ Program. 2009:385–395. doi:10.1182/asheducation-2009.1.385
de Jonge HJ, Valk PJ, Veeger NJ et al (2010) High VEGFC expression is associated with unique gene expression profiles and predicts adverse prognosis in pediatric and adult acute myeloid leukemia. Blood 116:1747–1754
Bullinger L, Dohner K, Bair E et al (2004) Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med 350:1605–1616
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4–25
Tammela T, Enholm B, Alitalo K, Paavonen K (2005) The biology of vascular endothelial growth factors. Cardiovasc Res 65:550–563
Joukov V, Sorsa T, Kumar V et al (1997) Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 16:3898–3911
Hou HA, Chou WC, Lin LI et al (2008) Expression of angiopoietins and vascular endothelial growth factors and their clinical significance in acute myeloid leukemia. Leuk Res 32:904–912
Padro T, Bieker R, Ruiz S et al (2002) Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR-2) in the bone marrow of patients with acute myeloid leukemia. Leukemia 16:1302–1310
Hiramatsu A, Miwa H, Shikami M et al (2006) Disease-specific expression of VEGF and its receptors in AML cells: possible autocrine pathway of VEGF/type1 receptor of VEGF in t(1517) AML and VEGF/type2 receptor of VEGF in t(821) AML. Leuk Lymphoma 47:89–95
Ter Elst A, Ma B, Scherpen FJ et al (2011) Repression of vascular endothelial growth factor expression by the runt-related transcription factor 1 in acute myeloid leukemia. Cancer Res 71:2761–2771
Chien MH, Ku CC, Johansson G et al (2009) Vascular endothelial growth factor-C (VEGF-C) promotes angiogenesis by induction of COX-2 in leukemic cells via the VEGF-R3/JNK/AP-1 pathway. Carcinogenesis 30:2005–2013
Fielder W, Graeven U, Ergun S et al (1997) Expression of FLT4 and its ligand VEGF-C in acute myeloid leukemia. Leukemia 11:1234–1237
Liersch R, Schliemann C, Bieker R et al (2008) Expression of VEGF-C and its receptor VEGFR-3 in the bone marrow of patients with acute myeloid leukaemia. Leuk Res 32:954–961
Kampen KR, ter Elst A, Mulder AB et al (2011) Anti-VEGFC treatment reduces the leukemic outgrowth of primary CD34+ pediatric acute myeloid leukemia cells. Blood, Suppl ASH abstract 4319
Ter Elst A, Kampen KR, Diks SH et al (2010) Targeting multiple active kinase pathways in 11q23 translocated pediatric acute myeloid leukemia using a VEGFR2 antibody together with a MEK inhibitor. Blood, Suppl ASH abstract 3626
Blazquez C, Cook N, Micklem K, Harris AL, Gatter KC, Pezzella F (2006) Phosphorylated KDR can be located in the nucleus of neoplastic cells. Cell Res 16:93–98
Santos SC, Dias S (2004) Internal and external autocrine VEGF/KDR loops regulate survival of subsets of acute leukemia through distinct signaling pathways. Blood 103:3883–3889
Dias S, Choy M, Alitalo K, Rafii S (2002) Vascular endothelial growth factor (VEGF)-C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood 99:2179–2184
Aguayo A, Kantarjian HM, Estey EH et al (2002) Plasma vascular endothelial growth factor levels have prognostic significance in patients with acute myeloid leukemia but not in patients with myelodysplastic syndromes. Cancer 95:1923–1930
De Bont ES, Fidler V, Meeuwsen T, Scherpen F, Hahlen K, Kamps WA (2002) Vascular endothelial growth factor secretion is an independent prognostic factor for relapse-free survival in pediatric acute myeloid leukemia patients. Clin Cancer Res 8:2856–2861
Wegiel B, Ekberg J, Talasila KM, Jalili S, Persson JL (2009) The role of VEGF and a functional link between VEGF and p27Kip1 in acute myeloid leukemia. Leukemia 23:251–261
Kruizinga RC, de Jonge HJ, Kampen KR, Walenkamp AM, De Bont ES (2011) Vascular endothelial growth Factor A isoform mRNA expression in pediatric acute myeloid leukemia. Pediatr Blood Cancer 56:294–297
Kim DH, Lee NY, Lee MH, Sohn SK, Do YR, Park JY (2008) Vascular endothelial growth factor (VEGF) gene (VEGFA) polymorphism can predict the prognosis in acute myeloid leukaemia patients. Br J Haematol 140:71–79
Monzo M, Brunet S, Urbano-Ispizua A et al (2006) Genomic polymorphisms provide prognostic information in intermediate-risk acute myeloblastic leukemia. Blood 107:4871–4879
Debrah AY, Mand S, Toliat MR et al (2007) Plasma vascular endothelial growth Factor-A (VEGF-A) and VEGF-A gene polymorphism are associated with hydrocele development in lymphatic filariasis. Am J Trop Med Hyg 77:601–608
Koukourakis MI, Papazoglou D, Giatromanolaki A, Bougioukas G, Maltezos E, Sivridis E (2004) VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer 46:293–298
Stevens A, Soden J, Brenchley PE, Ralph S, Ray DW (2003) Haplotype analysis of the polymorphic human vascular endothelial growth factor gene promoter. Cancer Res 63:812–816
de Jonge HJ, Weidenaar AC, Ter Elst A et al (2008) Endogenous vascular endothelial growth factor-C expression is associated with decreased drug responsiveness in childhood acute myeloid leukemia. Clin Cancer Res 14:924–930
Yip W, De G, Raby BA et al (2011) Identifying causal rare variants of disease through family-based analysis of Genetics Analysis Workshop 17 data set. BMC Proceedings 5(Suppl 9):S21. doi:10.1186/1753-6561-5-S9-S21
Hong JM, Kim TH, Kim HJ, Park EK, Yang EK, Kim SY (2010) Genetic association of angiogenesis- and hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp Mol Med 42:376–385
Foss B, Mentzoni L, Bruserud O (2001) Effects of vascular endothelial growth factor on acute myelogenous leukemia blasts. J Hematother Stem Cell Res 10:81–93
Coppola S, Narciso L, Feccia T et al (2006) Enforced expression of KDR receptor promotes proliferation, survival and megakaryocytic differentiation of TF1 progenitor cell line. Cell Death Differ 13:61–74
Dias S, Shmelkov SV, Lam G, Rafii S (2002) VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood 99:2532–2540
Flandrin P, Guyotat D, Duval A et al (2008) Significance of heat-shock protein (HSP) 90 expression in acute myeloid leukemia cells. Cell Stress Chaperones 13:357–364
Schepers H, Geugien M, van der Toorn M et al (2005) HSP27 protects AML cells against VP-16-induced apoptosis through modulation of p38 and c-Jun. Exp Hematol 33:660–670
Fragoso R, Elias AP, Dias S (2007) Autocrine VEGF loops, signaling pathways, and acute leukemia regulation. Leuk Lymphoma 48:481–488
Imai N, Shikami M, Miwa H et al (2006) t(821) acute myeloid leukaemia cells are dependent on vascular endothelial growth factor (VEGF)/VEGF receptor type2 pathway and phosphorylation of Akt. Br J Haematol 135:673–682
Imai N, Miwa H, Shikami M et al (2009) Growth inhibition of AML cells with specific chromosome abnormalities by monoclonal antibodies to receptors for vascular endothelial growth factor. Leuk Res 33:1650–1657
Gerhardt H, Golding M, Fruttiger M et al (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177
Hoeben A, Landuyt B, Highley MS, Wildiers H, van Oosterom AT, de Bruijn EA (2004) Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56:549–580
Smith NR, Baker D, James NH et al (2010) Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res 16:3548–3561
Gerber HP, McMurtrey A, Kowalski J et al (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273:30336–30343
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7:359–371
Hussong JW, Rodgers GM, Shami PJ (2000) Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood 95:309–313
De Bont ES, Rosati S, Jacobs S, Kamps WA, Vellenga E (2001) Increased bone marrow vascularization in patients with acute myeloid leukaemia: a possible role for vascular endothelial growth factor. Br J Haematol 113:296–304
Rabitsch W, Sperr WR, Lechner K et al (2004) Bone marrow microvessel density and its prognostic significance in AML. Leuk Lymphoma 45:1369–1373
Padro T, Ruiz S, Bieker R et al (2000) Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia. Blood 95:2637–2644
Weidenaar AC, Ter Elst A, Koopmans-Klein G et al (2011) High acute myeloid leukemia-derived VEGFA levels are associated with a specific vascular morphology in the leukemic bone marrow. Cell Oncol (Dordr) 34:289–296
Weidenaar AC, Ter Elst A, van Montfort CAGM et al (2011) Patterns of bone marrow micro vessel morphology in AML and high risk MDS predict treatment outcome following intensive chemotherapy and bevacizumab. Blood, Suppl ASH abstract 1555
Schuch G, Machluf M, Bartsch G Jr et al (2002) In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood 100:4622–4628
Spradling A, Drummond-Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414:98–104
Wilson A, Trumpp A (2006) Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6:93–106
Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121
Lo CC, Fleming HE, Wu JW et al (2009) Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457:92–96
Kubota Y, Takubo K, Suda T (2008) Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun 366:335–339
Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD (2007) Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 104:5431–5436
Kagiwada H, Yashiki T, Ohshima A, Tadokoro M, Nagaya N, Ohgushi H (2008) Human mesenchymal stem cells as a stable source of VEGF-producing cells. J Tissue Eng Regen Med 2:184–189
Wang M, Zhang W, Crisostomo P et al (2007) STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol 42:1009–1015
Deckers MM, van Bezooijen RL, van der Horst G et al (2002) Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 143:1545–1553
Kodama I, Niida S, Sanada M et al (2004) Estrogen regulates the production of VEGF for osteoclast formation and activity in op/op mice. J Bone Miner Res 19:200–206
Knowles HJ, Athanasou NA (2008) Hypoxia-inducible factor is expressed in giant cell tumour of bone and mediates paracrine effects of hypoxia on monocyte-osteoclast differentiation via induction of VEGF. J Pathol 215:56–66
Zhang Q, Guo R, Lu Y et al (2008) VEGF-C, a lymphatic growth factor, is a RANKL target gene in osteoclasts that enhances osteoclastic bone resorption through an autocrine mechanism. J Biol Chem 283:13491–13499
Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ (2011) Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood 117:1540–1549
Ishikawa F, Yoshida S, Saito Y et al (2007) Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25:1315–1321
Ninomiya M, Abe A, Katsumi A et al (2007) Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia 21:136–142
Zahiragic L, Schliemann C, Bieker R et al (2007) Bevacizumab reduces VEGF expression in patients with relapsed and refractory acute myeloid leukemia without clinical antileukemic activity. Leukemia 21:1310–1312
Karp JE, Gojo I, Pili R et al (2004) Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res 10:3577–3585
Roboz GJ, Giles FJ, List AF et al (2006) Phase 1 study of PTK787/ZK 222584, a small molecule tyrosine kinase receptor inhibitor, for the treatment of acute myeloid leukemia and myelodysplastic syndrome. Leukemia 20:952–957
Mesters RM, Padro T, Bieker R et al (2001) Stable remission after administration of the receptor tyrosine kinase inhibitor SU5416 in a patient with refractory acute myeloid leukemia. Blood 98:241–243
Fiedler W, Mesters R, Tinnefeld H et al (2003) A phase 2 clinical study of SU5416 in patients with refractory acute myeloid leukemia. Blood 102:2763–2767
Fiedler W, Serve H, Dohner H et al (2005) A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood 105:986–993
Giles FJ, Bellamy WT, Estrov Z et al (2006) The anti-angiogenesis agent, AG-013736, has minimal activity in elderly patients with poor prognosis acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Leuk Res 30:801–811
Metzelder S, Wang Y, Wollmer E et al (2009) Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood 113:6567–6571
Fischer T, Stone RM, Deangelo DJ et al (2010) Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol 28:4339–4345
Juckett M, LaPlant B, Flynn PJ et al (2011) Phase II study of AZD2171 for the treatment of patients with acute myelogenous leukemia. J Clin Oncol 29, ASCO, Suppl abstract 6574
Bruns AF, Herbert SP, Odell AF et al (2010) Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic 11:161–174
Acknowledgments
None.
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kampen, K.R., ter Elst, A. & de Bont, E.S.J.M. Vascular endothelial growth factor signaling in acute myeloid leukemia. Cell. Mol. Life Sci. 70, 1307–1317 (2013). https://doi.org/10.1007/s00018-012-1085-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1085-3