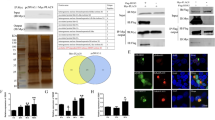
Abstract
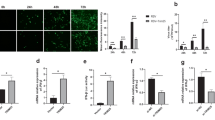
Coxsackievirus B3 (CVB3) is one of the most prevalent causes of viral myocarditis and is associated with many other pathological conditions. CVB3 replication relies on host cellular machineries and causes direct damage to host cells. MicroRNAs have been found to regulate viral infections but their roles in CVB3 infection are still poorly understood. Here we describe a novel mechanism by which miR-126 regulates two signal pathways essential for CVB3 replication. We found that CVB3-induced ERK1/2 activation triggered the phosphorylation of ETS-1 and ETS-2 transcription factors, which induced miR-126 upregulation. By using both microRNA mimics and inhibitors, we proved that the upregulated miR-126 suppressed sprouty-related, EVH1 domain containing 1 (SPRED1) and in turn enhanced ERK1/2 activation. This positive feedback loop of ERK1/2–miR-126–ERK1/2 promoted CVB3 replication. Meanwhile, miR-126 expression stimulated GSK-3β activity and induced degradation of β-catenin through suppressing LRP6 and WRCH1, two newly identified targets in the Wnt/β-catenin pathway, which sensitized the cells to virus-induced cell death and increased viral progeny release to initiate new infections. Our results demonstrate that upregulated miR-126 upon CVB3 infection targets SPRED1, LRP6, and WRCH1 genes, mediating cross-talk between ERK1/2 and Wnt/β-catenin pathways, and thus promoting viral replication and contributes to the viral cytopathogenicity.








Similar content being viewed by others
References
Granville DJ, Carthy CM, Yang D, Hunt DW, McManus BM (1998) Interaction of viral proteins with host cell death machinery. Cell Death Differ 5(8):653–659
Huber SA, Gauntt CJ, Sakkinen P (1998) Enteroviruses and myocarditis: viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv Virus Res 51:35–80
Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R (2004) Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med 141(11):829–834
Carthy CM, Granville DJ, Watson KA, Anderson DR, Wilson JE, Yang D, Hunt DW, McManus BM (1998) Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J Virol 72(9):7669–7675
Carthy CM, Yanagawa B, Luo H, Granville DJ, Yang D, Cheung P, Cheung C, Esfandiarei M, Rudin CM, Thompson CB, Hunt DW, McManus BM (2003) Bcl-2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology 313(1):147–157
Yuan JP, Zhao W, Wang HT, Wu KY, Li T, Guo XK, Tong SQ (2003) Coxsackievirus B3-induced apoptosis and caspase-3. Cell Res 13(3):203–209
Taylor LA, Carthy CM, Yang D, Saad K, Wong D, Schreiner G, Stanton LW, McManus BM (2000) Host gene regulation during coxsackievirus B3 infection in mice: assessment by microarrays. Circ Res 87(4):328–334
Zhang ZC, Li SJ, Yang YZ, Chen RZ, Ge JB, Chen HZ (2004) Microarray analysis of extracellular matrix genes expression in myocardium of mouse with Coxsackie virus B3 myocarditis. Chin Med J (Engl) 117(8):1228–1231
Rassmann A, Henke A, Zobawa M, Carlsohn M, Saluz HP, Grabley S, Lottspeich F, Munder T (2006) Proteome alterations in human host cells infected with coxsackievirus B3. J Gen Virol 87(Pt 9):2631–2638
Hammer E, Phong TQ, Steil L, Klingel K, Salazar MG, Bernhardt J, Kandolf R, Kroemer HK, Felix SB, Volker U (2010) Viral myocarditis induced by Coxsackievirus B3 in A.BY/SnJ mice: analysis of changes in the myocardial proteome. Proteomics 10(9):1802–1818
Luo H, Yanagawa B, Zhang J, Luo Z, Zhang M, Esfandiarei M, Carthy C, Wilson JE, Yang D, McManus BM (2002) Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J Virol 76(7):3365–3373
Cunningham KA, Chapman NM, Carson SD (2003) Caspase-3 activation and ERK phosphorylation during CVB3 infection of cells: influence of the coxsackievirus and adenovirus receptor and engineered variants. Virus Res 92(2):179–186
Marchant D, Sall A, Si X, Abraham T, Wu W, Luo Z, Petersen T, Hegele RG, McManus BM (2009) ERK MAP kinase-activated Arf6 trafficking directs coxsackievirus type B3 into an unproductive compartment during virus host-cell entry. J Gen Virol 90(Pt 4):854–862
Huber M, Watson KA, Selinka HC, Carthy CM, Klingel K, McManus BM, Kandolf R (1999) Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J Virol 73(5):3587–3594
Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, Miyoshi K, Tsuneoka M, Komiya S, Baron R, Yoshimura A (2001) Spred is a Sprouty-related suppressor of Ras signalling. Nature 412(6847):647–651
Yuan J, Zhang J, Wong BW, Si X, Wong J, Yang D, Luo H (2005) Inhibition of glycogen synthase kinase 3beta suppresses coxsackievirus-induced cytopathic effect and apoptosis via stabilization of beta-catenin. Cell Death Differ 12(8):1097–1106
Wu X, Quondamatteo F, Lefever T, Czuchra A, Meyer H, Chrostek A, Paus R, Langbein L, Brakebusch C (2006) Cdc42 controls progenitor cell differentiation and beta-catenin turnover in skin. Genes Dev 20(5):571–585
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5(7):522–531
Pasquinelli AE (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet 13(4):271–282
Sullivan CS, Ganem D (2005) MicroRNAs and viral infection. Mol Cell 20(1):3–7
Jangra RK, Yi M, Lemon SM (2010) Regulation of hepatitis C virus translation and infectious virus production by the microRNA miR-122. J Virol 84(13):6615–6625
Huang T, Zhang X (2012) Functional analysis of a crustacean microRNA in host-virus interactions. J Virol 86(23):12997–13004
Liu B, Peng XC, Zheng XL, Wang J, Qin YW (2009) MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer 66(2):169–175
Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D (2008) MiR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15(2):272–284
Small EM, Frost RJ, Olson EN (2010) MicroRNAs add a new dimension to cardiovascular disease. Circulation 121(8):1022–1032
Ye X, Liu Z, Hemida MG, Yang D (2011) Targeted delivery of mutant tolerant anti-coxsackievirus artificial microRNAs using folate conjugated bacteriophage Phi29 pRNA. PLoS One 6(6):e21215
Harris TA, Yamakuchi M, Kondo M, Oettgen P, Lowenstein CJ (2010) Ets-1 and Ets-2 regulate the expression of microRNA-126 in endothelial cells. Arter Thromb Vasc Biol 30(10):1990–1997
Foulds CE, Nelson ML, Blaszczak AG, Graves BJ (2004) Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol Cell Biol 24(24):10954–10964
Lim BK, Nam JH, Gil CO, Yun SH, Choi JH, Kim DK, Jeon ES (2005) Coxsackievirus B3 replication is related to activation of the late extracellular signal-regulated kinase (ERK) signal. Virus Res 113(2):153–157
Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP (2011) Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol 18(10):1139–1146
Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X (2008) Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135(2):367–375
Wu G, Huang H, Garcia Abreu J, He X (2009) Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One 4(3):e4926
Esfandiarei M, McManus BM (2008) Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol 3:127–155
Ho BC, Yu SL, Chen JJ, Chang SY, Yan BS, Hong QS, Singh S, Kao CL, Chen HY, Su KY, Li KC, Cheng CL, Cheng HW, Lee JY, Lee CN, Yang PC (2011) Enterovirus-induced miR-141 contributes to shutoff of host protein translation by targeting the translation initiation factor eIF4E. Cell Host Microbe 9(1):58–69
Morgan R, Anderson A, Bernberg E, Kamboj S, Huang E, Lagasse G, Isaacs G, Parcells M, Meyers BC, Green PJ, Burnside J (2008) Sequence conservation and differential expression of Marek’s disease virus microRNAs. J Virol 82(24):12213–12220
Esfandiarei M, Luo H, Yanagawa B, Suarez A, Dabiri D, Zhang J, McManus BM (2004) Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J Virol 78(8):4289–4298
Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E (2008) LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of beta-catenin. Proc Natl Acad Sci USA 105(23):8032–8037
Acknowledgments
This work was supported by a grant from Canadian Institute of Health Research (MOP231119).
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Ye and M. G. Hemida contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ye, X., Hemida, M.G., Qiu, Y. et al. MiR-126 promotes coxsackievirus replication by mediating cross-talk of ERK1/2 and Wnt/β-catenin signal pathways. Cell. Mol. Life Sci. 70, 4631–4644 (2013). https://doi.org/10.1007/s00018-013-1411-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1411-4