Abstract
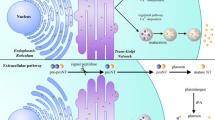
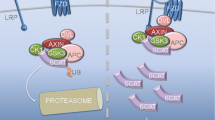
p75NTR, the common receptor for both neurotrophins and proneurotrophins, has been widely studied because of its role in many tissues, including the nervous system. More recently, a close relationship between p75NTR expression and pluripotency has been described. p75NTR was shown to be expressed in various types of stem cells and has been used to prospectively isolate stem cells with different degrees of potency. Here, we give an overview of the current knowledge on p75NTR in stem cells, ranging from embryonic to adult stem cells, and cancer stem cells. In an attempt to address its potential role in the control of stem cell biology, the molecular mechanisms underlying p75NTR signaling in different models are also highlighted. p75NTR-mediated functions include survival, apoptosis, migration, and differentiation, and depend on cell type, (pro)neurotrophin binding, interacting transmembrane co-receptors expression, intracellular adaptor molecule availability, and post-translational modifications, such as regulated proteolytic processing. It is therefore conceivable that p75NTR can modulate cell-fate decisions through its highly ramified signaling pathways. Thus, elucidating the potential implications of p75NTR activity as well as the underlying molecular mechanisms of p75NTR will shed new light on the biology of both normal and cancer stem cells.


Similar content being viewed by others
References
Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 78:7634–7638
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3:730–737
Chen Y, Zeng J, Cen L, Chen Y, Wang X, Yao G, Wang W, Qi W, Kong K (2009) Multiple roles of the p75 neurotrophin receptor in the nervous system. J Int Med Res 37:281–288
Schor NF (2005) The p75 neurotrophin receptor in human development and disease. Prog Neurobiol 77:201–214
Jiang X, Gwye Y, McKeown SJ, Bronner-Fraser M, Lutzko C, Lawlor ER (2009) Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev 18:1059–1070
Xiong J, Zhou L, Yang M, Lim Y, Zhu Y, Fu D, Li Z, Zhong J, Xiao Z, Zhou X-F (2013) ProBDNF and its receptors are upregulated in glioma and inhibit the growth of glioma cells in vitro. Neuro Oncol 15:990–1007
Guo J, Wang J, Liang C, Yan J, Wang Y, Liu G, Jiang Z, Zhang L, Wang X, Wang Y et al (2013) proNGF inhibits proliferation and oligodendrogenesis of postnatal hippocampal neural stem/progenitor cells through p75NTR in vitro. Stem Cell Res 11:874–887
Bartkowska K, Turlejski K, Djavadian RL (2010) Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol Exp (Wars) 70:454–467
He X-L, Garcia KC (2004) Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science 304:870–875
Large TH, Weskamp G, Helder JC, Radeke MJ, Misko TP, Shooter EM, Reichardt LF (1989) Structure and developmental expression of the nerve growth factor receptor in the chicken central nervous system. Neuron 2:1123–1134
Vilar M, Charalampopoulos I, Kenchappa RS, Simi A, Karaca E, Reversi A, Choi S, Bothwell M, Mingarro I, Friedman WJ et al (2009) Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers. Neuron 62:72–83
Coulson EJ, Reid K, Shipham KM, Morley S, Kilpatrick TJ, Bartlett PF (2004) The role of neurotransmission and the Chopper domain in p75 neurotrophin receptor death signaling. Prog Brain Res 146:41–62
Barker PA, Barbee G, Misko TP, Shooter EM (1994) The low affinity neurotrophin receptor, p75LNTR, is palmitoylated by thioester formation through cysteine 279. J Biol Chem 269:30645–30650
Skeldal S, Matusica D, Nykjaer A, Coulson EJ (2011) Proteolytic processing of the p75 neurotrophin receptor: a prerequisite for signalling?: neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75(NTR). BioEssays 33:614–625
Underwood CK, Coulson EJ (2008) The p75 neurotrophin receptor. Int J Biochem Cell Biol 40:1664–1668
Lu B, Pang PT, Woo NH (2005) The yin and yang of neurotrophin action. Nat Rev Neurosci 6:603–614
Gentry JJ, Casaccia-Bonnefil P, Carter BD (2000) Nerve growth factor activation of nuclear factor kappaB through its p75 receptor is an anti-apoptotic signal in RN22 schwannoma cells. J Biol Chem 275:7558–7565
Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE (1993) Induction of apoptosis by the low-affinity NGF receptor. Science 261:345–348
Yamashita T, Tucker KL, Barde YA (1999) Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 24:585–593
Notterpek L (2003) Neurotrophins in myelination: a new role for a puzzling receptor. Trends Neurosci 26:232–234
Chittka A, Arevalo JC, Rodriguez-Guzman M, Pérez P, Chao MV, Sendtner M (2004) The p75NTR-interacting protein SC1 inhibits cell cycle progression by transcriptional repression of cyclin E. J Cell Biol 164:985–996
Herrmann JL, Menter DG, Hamada J, Marchetti D, Nakajima M, Nicolson GL (1993) Mediation of NGF-stimulated extracellular matrix invasion by the human melanoma low-affinity p75 neurotrophin receptor: melanoma p75 functions independently of trkA. Mol Biol Cell 4:1205–1216
Johnston ALM, Lun X, Rahn JJ, Liacini A, Wang L, Hamilton MG, Parney IF, Hempstead BL, Robbins SM, Forsyth PA et al (2007) The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol 5:e212
Nakamura T, Endo K, Kinoshita S (2007) Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells 25:628–638
Vilar M, Charalampopoulos I, Kenchappa RS, Reversi A, Klos-Applequist JM, Karaca E, Simi A, Spuch C, Choi S, Friedman WJ et al (2009) Ligand-independent signaling by disulfide-crosslinked dimers of the p75 neurotrophin receptor. J Cell Sci 122:3351–3357
Barker PA (2004) p75NTR is positively promiscuous: novel partners and new insights. Neuron 42:529–533
Bronfman FC, Fainzilber M (2004) Multi-tasking by the p75 neurotrophin receptor: sortilin things out? EMBO Rep 5:867–871
Teng KK, Hempstead BL (2004) Neurotrophins and their receptors: signaling trios in complex biological systems. Cell Mol Life Sci 61:35–48
Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, Hempstead BL (2001) The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J Biol Chem 276:32687–32695
Iacaruso MF, Galli S, Martí M, Villalta JI, Estrin DA, Jares-Erijman EA, Pietrasanta LI (2011) Structural model for p75(NTR)-TrkA intracellular domain interaction: a combined FRET and bioinformatics study. J Mol Biol 414:681–698
Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE et al (2004) Sortilin is essential for proNGF-induced neuronal cell death. Nature 427:843–848
Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen Z-Y, Lee FS et al (2005) ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 25:5455–5463
Wang KC, Kim JA, Sivasankaran R, Segal R, He Z (2002) P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420:74–78
Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B et al (2004) LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci 7:221–228
Niederöst B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE (2002) Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci 22:10368–10376
Bertrand MJM, Kenchappa RS, Andrieu D, Leclercq-Smekens M, Nguyen HNT, Carter BD, Muscatelli F, Barker PA, De Backer O (2008) NRAGE, a p75NTR adaptor protein, is required for developmental apoptosis in vivo. Cell Death Differ 15:1921–1929
Mukai J, Hachiya T, Shoji-Hoshino S, Kimura MT, Nadano D, Suvanto P, Hanaoka T, Li Y, Irie S, Greene LA et al (2000) NADE, a p75NTR-associated cell death executor, is involved in signal transduction mediated by the common neurotrophin receptor p75NTR. J Biol Chem 275:17566–17570
Linggi MS, Burke TL, Williams BB, Harrington A, Kraemer R, Hempstead BL, Yoon SO, Carter BD (2005) Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J Biol Chem 280:13801–13808
Geetha T, Zheng C, McGregor WC, Douglas White B, Diaz-Meco MT, Moscat J, Babu JR (2012) TRAF6 and p62 inhibit amyloid β-induced neuronal death through p75 neurotrophin receptor. Neurochem Int 61:1289–1293
Ye X, Mehlen P, Rabizadeh S, VanArsdale T, Zhang H, Shin H, Wang JJ, Leo E, Zapata J, Hauser CA et al (1999) TRAF family proteins interact with the common neurotrophin receptor and modulate apoptosis induction. J Biol Chem 274:30202–30208
Khursigara G, Bertin J, Yano H, Moffett H, DiStefano PS, Chao MV (2001) A prosurvival function for the p75 receptor death domain mediated via the caspase recruitment domain receptor-interacting protein 2. J Neurosci 21:5854–5863
Irie S, Hachiya T, Rabizadeh S, Maruyama W, Mukai J, Li Y, Reed JC, Bredesen DE, Sato TA (1999) Functional interaction of Fas-associated phosphatase-1 (FAP-1) with p75(NTR) and their effect on NF-kappaB activation. FEBS Lett 460:191–198
Sole C, Dolcet X, Segura MF, Gutierrez H, Diaz-Meco M-T, Gozzelino R, Sanchis D, Bayascas JR, Gallego C, Moscat J et al (2004) The death receptor antagonist FAIM promotes neurite outgrowth by a mechanism that depends on ERK and NF-kappa B signaling. J Cell Biol 167:479–492
El Yazidi-Belkoura I, Adriaenssens E, Dollé L, Descamps S, Hondermarck H (2003) Tumor necrosis factor receptor-associated death domain protein is involved in the neurotrophin receptor-mediated antiapoptotic activity of nerve growth factor in breast cancer cells. J Biol Chem 278:16952–16956
Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M (2006) The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science 314:832–836
Sachs BD, Baillie GS, McCall JR, Passino MA, Schachtrup C, Wallace DA, Dunlop AJ, MacKenzie KF, Klussmann E, Lynch MJ et al (2007) p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol 177:1119–1132
Ito H, Morishita R, Iwamoto I, Mizuno M, Nagata K-I (2013) MAGI-1 acts as a scaffolding molecule for NGF receptor-mediated signaling pathway. Biochim Biophys Acta 1833:2302–2310
Epa WR, Markovska K, Barrett GL (2004) The p75 neurotrophin receptor enhances TrkA signalling by binding to Shc and augmenting its phosphorylation. J Neurochem 89:344–353
Underwood CK, Reid K, May LM, Bartlett PF, Coulson EJ (2008) Palmitoylation of the C-terminal fragment of p75(NTR) regulates death signaling and is required for subsequent cleavage by gamma-secretase. Mol Cell Neurosci 37:346–358
Kommaddi RP, Thomas R, Ceni C, Daigneault K, Barker PA (2011) Trk-dependent ADAM17 activation facilitates neurotrophin survival signaling. FASEB J 25:2061–2070
Wang L, Rahn JJ, Lun X, Sun B, Kelly JJP, Weiss S, Robbins SM, Forsyth PA, Senger DL (2008) Gamma-secretase represents a therapeutic target for the treatment of invasive glioma mediated by the p75 neurotrophin receptor. PLoS Biol 6:e289
Urra S, Escudero CA, Ramos P, Lisbona F, Allende E, Covarrubias P, Parraguez JI, Zampieri N, Chao MV, Annaert W et al (2007) TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal gamma-secretase-mediated release of the p75 intracellular domain. J Biol Chem 282:7606–7615
Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC (2003) Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci 23:5425–5436
Parkhurst CN, Zampieri N, Chao MV (2010) Nuclear localization of the p75 neurotrophin receptor intracellular domain. J Biol Chem 285:5361–5368
Verbeke S, Tomellini E, Dhamani F, Meignan S, Adriaenssens E, Bourhis XL (2013) Extracellular cleavage of the p75 neurotrophin receptor is implicated in its pro-survival effect in breast cancer cells. FEBS Lett 587(16):2591–2596
Salama-Cohen P, Arévalo M-A, Meier J, Grantyn R, Rodríguez-Tébar A (2005) NGF controls dendrite development in hippocampal neurons by binding to p75NTR and modulating the cellular targets of Notch. Mol Biol Cell 16:339–347
Zhou F-Q, Zhou J, Dedhar S, Wu Y-H, Snider WD (2004) NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 42:897–912
Arévalo JC, Chao MV (2005) Axonal growth: where neurotrophins meet Wnts. Curr Opin Cell Biol 17:112–115
David MD, Yeramian A, Duñach M, Llovera M, Cantí C, de Herreros AG, Comella JX, Herreros J (2008) Signalling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential beta-catenin phosphorylation. J Cell Sci 121:2718–2730
Nelson WJ, Nusse R (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483–1487
Chen B-Y, Wang X, Wang Z-Y, Wang Y-Z, Chen L-W, Luo Z-J (2013) Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J Neurosci Res 91:30–41
Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N (2000) Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 97:11307–11312
Pyle AD, Lock LF, Donovan PJ (2006) Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol 24:344–350
Moscatelli I, Pierantozzi E, Camaioni A, Siracusa G, Campagnolo L (2009) p75 neurotrophin receptor is involved in proliferation of undifferentiated mouse embryonic stem cells. Exp Cell Res 315:3220–3232
Kawamura K, Kawamura N, Sato W, Fukuda J, Kumagai J, Tanaka T (2009) Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology 150:3774–3782
Betters E, Liu Y, Kjaeldgaard A, Sundström E, García-Castro MI (2010) Analysis of early human neural crest development. Dev Biol 344:578–592
Crane JF, Trainor PA (2006) Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol 22:267–286
Le Douarin NM, Calloni GW, Dupin E (2008) The stem cells of the neural crest. Cell Cycle 7:1013–1019
Delfino-Machín M, Chipperfield TR, Rodrigues FSLM, Kelsh RN (2007) The proliferating field of neural crest stem cells. Dev Dyn 236:3242–3254
Dupin E, Sommer L (2012) Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol 366:83–95
Morrison SJ, White PM, Zock C, Anderson DJ (1999) Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 96:737–749
Kléber M, Lee H-Y, Wurdak H, Buchstaller J, Riccomagno MM, Ittner LM, Suter U, Epstein DJ, Sommer L (2005) Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol 169:309–320
Stemple DL, Anderson DJ (1992) Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 71:973–985
Sieber-Blum M (1998) Growth factor synergism and antagonism in early neural crest development. Biochem Cell Biol 76:1039–1050
Li H-Y, Say EHM, Zhou X-F (2007) Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells 25:2053–2065
Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ (2002) Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 35:657–669
Becker L, Kulkarni S, Tiwari G, Micci M-A, Pasricha PJ (2012) Divergent fate and origin of neurosphere-like bodies from different layers of the gut. Am J Physiol Gastrointest Liver Physiol 302:G958–G965
Chalazonitis A (2004) Neurotrophin-3 in the development of the enteric nervous system. Prog Brain Res 146:243–263
Okumura T, Shimada Y, Imamura M, Yasumoto S (2003) Neurotrophin receptor p75(NTR) characterizes human esophageal keratinocyte stem cells in vitro. Oncogene 22:4017–4026
Li X, Shen Y, Di B, Li J, Geng J, Lu X, He Z (2012) Biological and clinical significance of p75NTR expression in laryngeal squamous epithelia and laryngocarcinoma. Acta Otolaryngol 132:314–324
Truzzi F, Marconi A, Atzei P, Panza MC, Lotti R, Dallaglio K, Tiberio R, Palazzo E, Vaschieri C, Pincelli C (2011) p75 neurotrophin receptor mediates apoptosis in transit-amplifying cells and its overexpression restores cell death in psoriatic keratinocytes. Cell Death Differ 18:948–958
Di Girolamo N, Sarris M, Chui J, Cheema H, Coroneo MT, Wakefield D (2008) Localization of the low-affinity nerve growth factor receptor p75 in human limbal epithelial cells. J Cell Mol Med 12:2799–2811
Marynka-Kalmani K, Treves S, Yafee M, Rachima H, Gafni Y, Cohen MA, Pitaru S (2010) The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells 28:984–995
Botchkarev VA, Botchkareva NV, Albers KM, Chen LH, Welker P, Paus R (2000) A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. FASEB J 14:1931–1942
Botchkarev VA, Yaar M, Gilchrest BA, Paus R (2003) p75 Neurotrophin receptor antagonist retards apoptosis-driven hair follicle involution (catagen). J Invest Dermatol 120:168–169
Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U, Zimmermann DR, Dufour S, Thiery JP, Meijer D et al (2006) Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol 175:1005–1015
Lambiase A, Aloe L, Mantelli F, Sacchetti M, Perrella E, Bianchi P, Rocco ML, Bonini S (2012) Capsaicin-induced corneal sensory denervation and healing impairment are reversed by NGF treatment. Invest Ophthalmol Vis Sci 53:8280–8287
Martens W, Wolfs E, Struys T, Politis C, Bronckaers A, Lambrichts I (2012) Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs 196(6):490–500
Mikami Y, Ishii Y, Watanabe N, Shirakawa T, Suzuki S, Irie S, Isokawa K, Honda MJ (2011) CD271/p75(NTR) inhibits the differentiation of mesenchymal stem cells into osteogenic, adipogenic, chondrogenic, and myogenic lineages. Stem Cells Dev 20:901–913
Wen X, Liu L, Deng M, Zhang L, Liu R, Xing Y, Zhou X, Nie X (2012) Characterization of p75(+) ectomesenchymal stem cells from rat embryonic facial process tissue. Biochem Biophys Res Commun 427:5–10
Stevens A, Zuliani T, Olejnik C, LeRoy H, Obriot H, Kerr-Conte J, Formstecher P, Bailliez Y, Polakowska RR (2008) Human dental pulp stem cells differentiate into neural crest-derived melanocytes and have label-retaining and sphere-forming abilities. Stem Cells Dev 17:1175–1184
Luukko K, Moshnyakov M, Sainio K, Saarma M, Sariola H, Thesleff I (1996) Expression of neurotrophin receptors during rat tooth development is developmentally regulated, independent of innervation, and suggests functions in the regulation of morphogenesis and innervation. Dev Dyn 206:87–99
Mitsiadis TA, Couble P, Dicou E, Rudkin BB, Magloire H (1993) Patterns of nerve growth factor (NGF), proNGF, and p75 NGF receptor expression in the rat incisor: comparison with expression in the molar. Differentiation 54:161–175
Cragnolini AB, Huang Y, Gokina P, Friedman WJ (2009) Nerve growth factor attenuates proliferation of astrocytes via the p75 neurotrophin receptor. Glia 57:1386–1392
Ibáñez CF, Simi A (2012) p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci 35:431–440
Young KM, Merson TD, Sotthibundhu A, Coulson EJ, Bartlett PF (2007) p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci 27:5146–5155
Du Y, Fischer TZ, Clinton-Luke P, Lercher LD, Dreyfus CF (2006) Distinct effects of p75 in mediating actions of neurotrophins on basal forebrain oligodendrocytes. Mol Cell Neurosci 31:366–375
Hapner SJ, Boeshore KL, Large TH, Lefcort F (1998) Neural differentiation promoted by truncated trkC receptors in collaboration with p75(NTR). Dev Biol 201:90–100
Bibel M, Richter J, Schrenk K, Tucker KL, Staiger V, Korte M, Goetz M, Barde Y-A (2004) Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat Neurosci 7:1003–1009
Nikoletopoulou V, Plachta N, Allen ND, Pinto L, Götz M, Barde Y-A (2007) Neurotrophin receptor-mediated death of misspecified neurons generated from embryonic stem cells lacking Pax6. Cell Stem Cell 1:529–540
Kendall SE, Ryczko MC, Mehan M, Verdi JM (2003) Characterization of NADE, NRIF and SC-1 gene expression during mouse neurogenesis. Brain Res Dev Brain Res 144:151–158
Lu J, Frerich JM, Turtzo LC, Li S, Chiang J, Yang C, Wang X, Zhang C, Wu C, Sun Z et al (2013) Histone deacetylase inhibitors are neuroprotective and preserve NGF-mediated cell survival following traumatic brain injury. Proc Natl Acad Sci USA 110:10747–10752
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Thomson TM, Rettig WJ, Chesa PG, Green SH, Mena AC, Old LJ (1988) Expression of human nerve growth factor receptor on cells derived from all three germ layers. Exp Cell Res 174:533–539
Maeda S, Nobukuni T, Shimo-Onoda K, Hayashi K, Yone K, Komiya S, Inoue I (2002) Sortilin is upregulated during osteoblastic differentiation of mesenchymal stem cells and promotes extracellular matrix mineralization. J Cell Physiol 193:73–79
Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL (2002) Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol 30:783–791
Yuan J, Huang G, Xiao Z, Lin L, Han T (2013) Overexpression of β-NGF promotes differentiation of bone marrow mesenchymal stem cells into neurons through regulation of AKT and MAPK pathway. Mol Cell Biochem 383(1–2):201–211
Yamamoto N, Akamatsu H, Hasegawa S, Yamada T, Nakata S, Ohkuma M, Miyachi E-I, Marunouchi T, Matsunaga K (2007) Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci 48:43–52
Rada T, Reis RL, Gomes ME (2011) Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev 7:64–76
Russo MA, Giustizieri ML, Favale A, Fantini MC, Campagnolo L, Konda D, Germano F, Farini D, Manna C, Siracusa G (1999) Spatiotemporal patterns of expression of neurotrophins and neurotrophin receptors in mice suggest functional roles in testicular and epididymal morphogenesis. Biol Reprod 61:1123–1132
Russo MA, Giustizieri ML, Farini D, Campagnolo L, De Felici M, Siracusa G (1996) Expression of the p75 neurotrophin receptor in the developing and adult testis of the rat. Int J Dev Biol Suppl 1:227S–228S
Zhang L, Wang H, Yang Y, Liu H, Zhang Q, Xiang Q, Ge R, Su Z, Huang Y (2013) NGF induces adult stem Leydig cells to proliferate and differentiate during Leydig cell regeneration. Biochem Biophys Res Commun 436:300–305
Colombo E, Romaggi S, Medico E, Menon R, Mora M, Falcone C, Lochmüller H, Confalonieri P, Mantegazza R, Morandi L et al (2011) Human neurotrophin receptor p75NTR defines differentiation-oriented skeletal muscle precursor cells: implications for muscle regeneration. J Neuropathol Exp Neurol 70:133–142
Colombo E, Bedogni F, Lorenzetti I, Landsberger N, Previtali SC, Farina C (2013) Autocrine and immune cell-derived BDNF in human skeletal muscle: implications for myogenesis and tissue regeneration. J Pathol 231:190–198
Mousavi K, Jasmin BJ (2006) BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J Neurosci 26:5739–5749
Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106:12771–12775
Geerts A (2004) On the origin of stellate cells: mesodermal, endodermal or neuro-ectodermal? J Hepatol 40:331–334
Zaret KS (2001) Hepatocyte differentiation: from the endoderm and beyond. Curr Opin Genet Dev 11:568–574
Cassiman D, Denef C, Desmet VJ, Roskams T (2001) Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology 33:148–158
Kendall TJ, Hennedige S, Aucott RL, Hartland SN, Vernon MA, Benyon RC, Iredale JP (2009) p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology 49:901–910
Passino MA, Adams RA, Sikorski SL, Akassoglou K (2007) Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science 315:1853–1856
Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M (2005) A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65:9328–9337
Dirks P (2010) Cancer stem cells: invitation to a second round. Nature 466:40–41
Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, Pincelli C (2008) Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol 128:2031–2040
Denkins Y, Reiland J, Roy M, Sinnappah-Kang ND, Galjour J, Murry BP, Blust J, Aucoin R, Marchetti D (2004) Brain metastases in melanoma: roles of neurotrophins. Neuro Oncol 6:154–165
Marchetti D, McQuillan DJ, Spohn WC, Carson DD, Nicolson GL (1996) Neurotrophin stimulation of human melanoma cell invasion: selected enhancement of heparanase activity and heparanase degradation of specific heparan sulfate subpopulations. Cancer Res 56:2856–2863
Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ et al (2010) Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 466:133–137
Eichhoff OM, Weeraratna A, Zipser MC, Denat L, Widmer DS, Xu M, Kriegl L, Kirchner T, Larue L, Dummer R et al (2011) Differential LEF1 and TCF4 expression is involved in melanoma cell phenotype switching. Pigment Cell Melanoma Res 24:631–642
Touil Y, Zuliani T, Wolowczuk I, Kuranda K, Prochazkova J, Andrieux J, Le Roy H, Mortier L, Vandomme J, Jouy N et al (2012) The PI3K/AKT signaling pathway controls the quiescence of the low-Rhodamine123-retention cell compartment enriched for melanoma stem cell activity. Stem Cells 31:641–651
Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M (2010) A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 141:583–594
Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M et al (2011) Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res 71:3098–3109
Søland TM, Brusevold IJ, Koppang HS, Schenck K, Bryne M (2008) Nerve growth factor receptor (p75 NTR) and pattern of invasion predict poor prognosis in oral squamous cell carcinoma. Histopathology 53:62–72
Kiyosue T, Kawano S, Matsubara R, Goto Y, Hirano M, Jinno T, Toyoshima T, Kitamura R, Oobu K, Nakamura S (2011) Immunohistochemical location of the p75 neurotrophin receptor (p75NTR) in oral leukoplakia and oral squamous cell carcinoma. Int J Clin Oncol 18(1):154–163
Lewis Kelso R, Colome-Grimmer MI, Uchida T, Wang HQ, Wagner RF Jr (2006) p75(NGFR) immunostaining for the detection of perineural invasion by cutaneous squamous cell carcinoma. Dermatol Surg 32:177–183
Okumura T, Tsunoda S, Mori Y, Ito T, Kikuchi K, Wang TC, Yasumoto S, Shimada Y (2006) The biological role of the low-affinity p75 neurotrophin receptor in esophageal squamous cell carcinoma. Clin Cancer Res 12:5096–5103
Huang S-D, Yuan Y, Liu X-H, Gong D-J, Bai C-G, Wang F, Luo J-H, Xu Z-Y (2009) Self-renewal and chemotherapy resistance of p75NTR-positive cells in esophageal squamous cell carcinomas. BMC Cancer 9:9
Demont Y, Corbet C, Page A, Ataman-Önal Y, Choquet-Kastylevsky G, Fliniaux I, Le Bourhis X, Toillon R-A, Bradshaw RA, Hondermarck H (2012) Pro-nerve growth factor induces autocrine stimulation of breast cancer cell invasion through tropomyosin-related kinase A (TrkA) and sortilin protein. J Biol Chem 287:1923–1931
Vanhecke E, Adriaenssens E, Verbeke S, Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X, Hondermarck H (2011) Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin Cancer Res 17:1741–1752
Descamps S, Pawlowski V, Révillion F, Hornez L, Hebbar M, Boilly B, Hondermarck H, Peyrat JP (2001) Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res 61:4337–4340
Descamps S, Lebourhis X, Delehedde M, Boilly B, Hondermarck H (1998) Nerve growth factor is mitogenic for cancerous but not normal human breast epithelial cells. J Biol Chem 273:16659–16662
Dollé L, Oliveira M-J, Bruyneel E, Hondermarck H, Bracke M (2005) Nerve growth factor mediates its pro-invasive effect in parallel with the release of a soluble E-cadherin fragment from breast cancer MCF-7/AZ cells. J Dairy Res 72 Spec No: 20–26
Popnikolov NK, Cavone SM, Schultz PM, Garcia FU (2005) Diagnostic utility of p75 neurotrophin receptor (p75NTR) as a marker of breast myoepithelial cells. Mod Pathol 18:1535–1541
Verbeke S, Meignan S, Lagadec C, Germain E, Hondermarck H, Adriaenssens E, Le Bourhis X (2010) Overexpression of p75(NTR) increases survival of breast cancer cells through p21(waf1). Cell Signal 22:1864–1873
Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP, Hondermarck H (2001) Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem 276:17864–17870
Kim J, Villadsen R, Sørlie T, Fogh L, Grønlund SZ, Fridriksdottir AJ, Kuhn I, Rank F, Wielenga VT, Solvang H et al (2012) Tumor initiating but differentiated luminal-like breast cancer cells are highly invasive in the absence of basal-like activity. Proc Natl Acad Sci USA 109:6124–6129
Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ (2007) A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev 21:3238–3243
Imai T, Tamai K, Oizumi S, Oyama K, Yamaguchi K, Sato I, Satoh K, Matsuura K, Saijo S, Sugamura K et al (2013) CD271 defines a stem cell-like population in hypopharyngeal cancer. PLoS ONE 8:e62002
Biagiotti T, D’Amico M, Marzi I, Di Gennaro P, Arcangeli A, Wanke E, Olivotto M (2006) Cell renewing in neuroblastoma: electrophysiological and immunocytochemical characterization of stem cells and derivatives. Stem Cells 24:443–453
Higuchi H, Yamashita T, Yoshikawa H, Tohyama M (2003) PKA phosphorylates the p75 receptor and regulates its localization to lipid rafts. EMBO J 22:1790–1800
Lee MY, Ryu JM, Lee SH, Park JH, Han HJ (2010) Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. J Lipid Res 51:2082–2089
Yamazaki S, Iwama A, Morita Y, Eto K, Ema H, Nakauchi H (2007) Cytokine signaling, lipid raft clustering, and HSC hibernation. Ann N Y Acad Sci 1106:54–63
Lu P, Jones LL, Snyder EY, Tuszynski MH (2003) Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol 181:115–129
Campagnolo L, Russo MA, Puglianiello A, Favale A, Siracusa G (2001) Mesenchymal cell precursors of peritubular smooth muscle cells of the mouse testis can be identified by the presence of the p75 neurotrophin receptor. Biol Reprod 64:464–472
Acknowledgments
This research was supported by the Institut National de la Santé et de la Recherche Médicale (Inserm), The Université de Lille 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomellini, E., Lagadec, C., Polakowska, R. et al. Role of p75 neurotrophin receptor in stem cell biology: more than just a marker. Cell. Mol. Life Sci. 71, 2467–2481 (2014). https://doi.org/10.1007/s00018-014-1564-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1564-9