Abstract
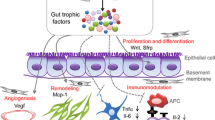
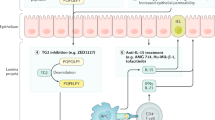
Despite the growing understanding of its pathogenesis, the treatment of coeliac disease is still based on a lifelong gluten-free diet that, although efficacious, is troublesome for affected patients, and a definitive cure is still an unmet need. In this regard, the development of new chemical- and biological-derived agents has often resulted in unsatisfactory effects when tested in vivo, probably because of their ability to target only a single pathway, whilst the immunological cascade responsible for tissue injury is complex and redundant. The advent of cellular therapies, mainly based on the use of stem cells, is an emerging area of interest since it has the advantage of a multi-target strategy. Both haematopoietic and mesenchymal stem cells have been employed in the treatment of refractory patients suffering from autoimmune diseases, with promising results. However, the lack of immunogenicity makes mesenchymal stem cells more suitable than their haematopoietic counterpart, since their transplantation may be performed in the absence of a myeloablative conditioning regimen. In addition, mesenchymal stem cells have been shown to harbour strong modulatory effects on almost all cells involved in immune response, together with a potent regenerative action. It is therefore conceivable that over the next few years their therapeutic use will increase as their biological interactions with injured tissues become clearer.


Similar content being viewed by others
Abbreviations
- CD:
-
Coeliac disease
- EATL:
-
Enteropathy-associated T cell lymphoma
- Fox:
-
Transcription factor Forkhead box
- GFD:
-
Gluten-free diet
- HSC:
-
Haematopoietic stem cell
- HGF:
-
Hepatocyte growth factor
- HLA:
-
Histocompatibility locus antigen
- IDO:
-
Indoleamine 2,3-dioxygenase
- IFN:
-
Interferon
- IL:
-
Interleukin
- IEL:
-
Intraepithelial lymphocyte
- JAK:
-
Janus kinase
- MSC:
-
Mesenchymal stem cells
- NO:
-
Nitric oxide
- PGE2 :
-
Prostaglandin E2
- STAT:
-
Signal transducer and activator of transcription
- TGF:
-
Transforming growth factor
- TNF:
-
Tumour necrosing factor
- VEGF:
-
Vascular endothelial growth factor
References
Okamoto R, Watanabe M (2004) Molecular and clinical basis for the regeneration of human gastrointestinal epithelia. J Gastroenterol 39:1–6
Koboziev I, Karlsson F, Grisham MB (2010) Gut-associated lymphoid tissue, T cell trafficking, and chronic intestinal inflammation. Ann NY Acad Sci 1207:E86–E93
Sollid LM, Qiao SW, Anderson RP et al (2012) Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics 64:455–460
Dieterich W, Ehnis T, Bauer M et al (1997) Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 3:797–801
Di Sabatino A, Corazza GR (2009) Coeliac disease. Lancet 373:1480–1493
Abadie V, Sollid LM, Barreiro LB et al (2011) Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol 29:493–525
Van de Kamer J, Weijers H, Dicke W (1953) Coeliac disease. Some experiments on the cause of the harmful effect of wheat gliadin. Acta Paediatr Scand 42:223–231
Lee AR, Ng DL, Diamond B et al (2012) Living with coeliac disease: survey results from the U.S.A. J Hum Nutr Diet 25:233–238
Biagi F, Gobbi P, Marchese A et al (2014) Low incidence but poor prognosis of complicated coeliac disease: a retrospective multicentre study. Dig Liver Dis 46:227–230
Marietta EV, Murray JA (2012) Animal models to study gluten sensitivity. Semin Immunopathol 34:497–511
Kaukinen K, Lindfors K, Maki M (2014) Advances in the treatment of coeliac disease: an immunopathogenic perspective. Nat Rev Gastroenterol Hepatol 11:36–44
Dazzi F, van Laar JM, Cope A et al (2007) Cell therapy for autoimmune diseases. Arthritis Res Ther. 9:206. doi:10.1186/ar2128
Burt RK, Loh Y, Pearce W et al (2008) Clinical applications of blood-derived and marrow-derived stem cells for non-malignant diseases. JAMA 299:925–936
Farge D, Labopin M, Tyndall A et al (2010) Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica 95:284–292
Andoh A, Bamba S, Fujiyama Y et al (2005) Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol 40:1089–1099
Brittan M, Chance V, Elia G et al (2005) A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology 128:1984–1995
Khalil PN, Weiler V, Nelson PJ et al (2007) Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology 132:944–954
Matsumoto T, Okamoto R, Yajima T et al (2005) Increase of bone marrow-derived secretory lineage epithelial cells during regeneration in the human intestine. Gastroenterology 128:1851–1867
Mastrandrea F, Semeraro FP, Coradduzza G et al (2008) CD34+ hemopoietic precursor and stem cells traffic in peripheral blood of celiac patients is significantly increased but not directly related to epithelial damage severity. Eur Ann Allergy Clin Immunol 40:90–103
Ciccocioppo R, Di Sabatino A, Parroni R et al (2001) Increased enterocyte apoptosis and Fas-Fas ligand system in celiac disease. Am J Clin Pathol 115:494–503
Burt RK, Testori A, Craig R et al (2008) Hematopoietic stem cell transplantation for autoimmune diseases: what have we learned? J Autoimmun 30:116–120
de Kleer I, Vastert B, Klein M et al (2006) Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood 107:1696–1702
Al-Toma A, Mulder CJJ (2007) Stem cell transplantation for the treatment of gastrointestinal diseases—current applications and future perspectives. Aliment Pharmacol Ther 26(Suppl. 2):77–89
Al-Toma A, Visser O, van Roessel HM et al (2007) Autologous hematopoietic stem cell transplantation in refractory celiac with aberrant T-cells. Blood 109:2243–2249
Tack GJ, Wondergem MJ, Al-Toma A et al (2011) Auto-SCT in refractory celiac disease type II patients unresponsive to cladribine therapy. Bone Marrow Transplant 46:840–846
Al-Toma A, Verbeek WH, Visser OJ et al (2007) Disappointing outcome of autologous stem cell transplantation for enteropathy-associated T-cell lymphoma. Dig Liver Dis 39:634–641
Malamut G, Cellier C (2013) Refractory coeliac disease. Curr Opin Oncol 25:445–451
Verkarre V, Romana SP, Cellier C et al (2003) Recurrent partial trisomy 1q22-q44 in clonal intraepithelial lymphocytes in refractory celiac sprue. Gastroenterology 125:40–46
Cellier C, Patey N, Mauvieux L et al (1998) Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology 114:471–481
Schmitz F, Tjon JM, Lai Y et al (2013) Identification of a potential physiological precursor of aberrant cells in refractory coeliac disease type II. Gut 62:509–519
Malamut G, Machhour R, Montcuquet N et al (2010) IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest 120:2131–2143
DePaolo RW, Abadie V, Tang F et al (2011) Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 471:220–224
Zanzi D, Stefanile R, Santagata S et al (2011) IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in celiac disease. Am J Gastroenterol 106:1308–1317
Hmida NB, Ben Ahmed M, Moussa A et al (2012) Impaired control of effector T cells by regulatory T cells: a clue to loss of oral tolerance and autoimmunity in celiac disease? Am J Gatroenterol. 107:604–611
Korneychuk N, Ramiro-Puig E, Ettersperger J et al (2014) Interleukin 15 and CD4+ T Cells cooperate to promote small intestinal enteropathy in response to dietary antigen. Gastroenterology 146:1017–1027
Kline RM, Neudorf SM, Baron HI (2007) Correction of celiac disease after allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia. Pediatrics 120:e1120–e1122
Hoekstra JH, Groot-Loonen JJ, van der Weij A et al (2010) Successful treatment of celiac disease by allogeneic haematopoietic stem cell transplantation. J Pediatr Gastroenterol Nutr 51:793–794
Ciccocioppo R, Bernardo ME, Russo ML et al (2013) Allogeneic hematopoietic stem cell transplantation may restore gluten tolerance in patients with celiac disease. J Pediatr Gastroenterol Nutr 56:422–427
Ben-Horin S, Polak-Charcon S, Barshack I et al (2013) Celiac disease resolution after allogeneic bone marrow transplantation is associated with absence of gliadin-specific memory response by donor-derived intestinal T-cells. J Clin Immunol 33:1395–1402
Friedenstein AJ, Gorskaja JF, Kulagina NN (1976) Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267–274
Ding D-C, Shyu W-C, Lin S-Z (2011) Mesenchymal stem cells. Cell Transplant 20:5–14
Charbord P (2010) Bone marrow mesenchymal stem cells: historical overview and concepts. Human Gene Ther 21:1045–1056
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Jiang Y, Jahagirdar BN, Reinhardt RL et al (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49
Bernardo ME, Locatelli F, Fibbe WE (2009) Mesenchymal stromal cells. Ann NY Acad Sci 1176:101–117
Wei Y, Nie Y, Lai J et al (2009) Comparison of the population capacity of hematopoietic and mesenchymal stem cells in experimental colitis rat model. Transplantation 88:42–48
Yabana T, Arimura Y, Tanaka H et al (2009) Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. J Pathol 218:350–359
Le Blanc K, Tammik C, Rosendahl K et al (2003) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 31:890–896
Majumdar MK, Keane-Moore M, Buyaner D et al (2003) Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 10:228–241
Barry FP, Murphy JM, English K, Mahon BP (2005) Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev 14:252–265
Sundin M, Ringdén O, Sundberg B et al (2007) No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica 92:1208–1215
De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A et al (2012) Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 12:574–591
Burr S, Dazzi F, Garden OA (2013) Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol 9:12–18
Gonzalez MA, Gonzalez-Rey E, Rico L et al (2009) Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 136:978–989
Prockop DJ, Kota DJ, Bazhanov N et al (2010) Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J Cell Mol Med 14:2190–2199
Siegel G, Schäfer R, Dazzi F (2009) The immunosuppressive properties of mesenchymal stem cells. Transplantation 87(9 Suppl):S45–S49
Fournel S, Aguerre-Girr M, Huc X et al (2000) Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol 164:6100–6104
Riteau B, Menier C, Khalil-Daher I et al (2001) HLA-G1 co-expression boosts the HLA class I-mediated NK lysis inhibition. Int Immunol 13:193–201
Selmani Z, Naji A, Zidi I et al (2008) Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 26:212–222
Ketroussi F, Giuliani M, Bahri R et al (2011) Lymphocyte cell-cycle inhibition by HLA-G is mediated by phosphatase SHP-2 and acts on the mTOR pathway. PLoS ONE 6:e22776
Ristich V, Liang S, Zhang W et al (2005) Tolerization of dendritic cells by HLA-G. Eur J Immunol 35:1133–1142
Le Blanc K, Frassoni F, Ball L et al (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579–1586
Parekkedan B, Tilles AW, Yarmush ML (2008) Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells 26:1913–1919
Ciccocioppo R, Russo ML, Bernardo ME et al (2012) Mesenchymal stromal cell infusions as rescue therapy for corticosteroid-refractory adult autoimmune enteropathy. Mayo Clin Proc 87:909–914
Meresse B, Malamut G, Cerf-Bensussan N (2012) Celiac disease: an immunological jigsaw. Immunity 36:907–919
Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153
Kyung JM, Klaus C, Kaemmerer E et al (2013) Intestinal barrier: molecular pathways and modifiers. World J Gastroenterol 4:94–99
Ciccocioppo R, Finamore A, Ara C et al (2006) Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am J Clin Pathol 125:502–511
Drago S, El Asmar R, Di Pierro M et al (2006) Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 41:408–419
Clemente MG, De Virgiliis S, Kang JS et al (2003) Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 52:218–223
Paterson BM, Lammers KM, Arrieta MC et al (2007) The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther 26:757–766
Leffler DA, Kelly CP, Abdallah HZ et al (2012) A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol 107:1554–1562
Kelly CP, Green PH, Murray JA et al (2013) Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther 37:252–262
Sémont A, Mouiseddine M, François A et al (2010) Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ 17:952–961
Weil BR, Markel TA, Herrmann JL et al (2009) Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery 146:190–197
Tayman C, Uckan D, Kilic E et al (2011) Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res 70:489–494
Cheroutre H, Lambolez F, Mucida D (2011) The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 11:445–456
Di Sabatino A, Ciccocioppo R, D’Alò S et al (2001) Intraepithelial and lamina propria lymphocytes show distinct patterns of apoptosis whereas both populations are active in Fas based cytotoxicity in coeliac disease. Gut 49:380–386
Olaussen RW, Johansen FE, Lundin KE et al (2002) Interferon-gamma-secreting T cells localize to the epithelium in coeliac disease. Scand J Immunol 56:652–664
Ciccocioppo R, Di Sabatino A, Parroni R et al (2000) Cytolytic mechanisms of intraepithelial lymphocytes in coeliac disease (CoD). Clin Exp Immunol 120:235–240
Meresse B, Chen Z, Ciszewski C et al (2004) Coordinated induction by IL-15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21:357–366
Sotiropoulou PA, Perez SA, Gritzapis AD et al (2006) Interaction between human mesenchymal stem cells and natural killer cells. Stem Cells 24:74–85
Spaggiari GM, Capobianco A, Becchetti S et al (2006) Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 107:1484–1490
Spaggiari GM, Capobianco A, Abdelrazik H et al (2008) Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111:1327–1333
Krampera M, Cosmi L, Angeli R et al (2006) Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24:386–398
Ciccocioppo R, Camarca A, Cangemi GC et al (2014) Tolerogenic effect of mesenchymal stromal cells on gliadin-specific T lymphocytes in celiac disease. Cytotherapy 16:1080–1091
Prigione I, Benvenuto F, Bocca P et al (2009) Reciprocal interactions between human mesenchymal stem cells and γö T cells or invariant Natural Killer T cells. Stem Cells 27:693–702
Dunne MR, Elliott L, Hussey S et al (2013) Persistent changes in circulating and intestinal γδ T cell subsets, invariant natural killer T cells and mucosal-associated invariant T cells in children and adults with coeliac disease. PLoS ONE 8:e76008. doi:10.1371/journal.pone.0076008
Sollid LM, Markussen G, Ek J et al (1989) Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med 169:345–350
Shan L, Molberg O, Parrot I et al (2002) Structural basis for gluten intolerance in celiac sprue. Science 297:2275–2279
Beitnes AC, Ráki M, Brottveit M et al (2012) Rapid accumulation of CD14+CD11c+ dendritic cells in gut mucosa of celiac disease after in vivo gluten challenge. PLoS ONE 7(3):e33556. doi:10.1371/journal.pone.0033556
Jung YJ, Ju SY, Yoo ES et al (2007) MSC-DC interactions: MSC inhibit maturation and migration of BM-derived DC. Cytotherapy 9:451–458
Spaggiari GM, Abdelrazik H, Becchetti F et al (2009) MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 113:6576–6583
English K, Barry FP, Mahon BP (2008) Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett 115:50–58
Ramasamy R, Fazekasova H, Lam EW et al (2007) Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 83:71–76
Nauta AJ, Kruisselbrink AB, Lurvink E et al (2006) Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol 177:2080–2087
Li Y-P, Paczesny S, Lauret E et al (2008) Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J Immunol 180:1598–1608
Zhang B, Liu R, Shi D et al (2009) Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood 113:46–57
Wang Q, Sun B, Wang D et al (2008) Murine bone marrow mesenchymal stem cells cause mature dendritic cells to promote T-cell tolerance. Scand J Immunol 68:607–615
Bai L, Lennon DP, Eaton V et al (2009) Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 57:1192–1203
Krampera M, Glennie S, Dyson J et al (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101:3722–3729
Di Nicola M, Carlo-Stella C, Magni M et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843
Sato K, Ozaki K, Oh I et al (2007) Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109:228–234
Fina D, Sarra M, Caruso R et al (2008) Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut 57:887–892
Bodd M, Ráki M, Tollefsen S et al (2010) HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol 3:594–601. doi:10.1038/mi.2010.36
Croitoru-Lamoury J, Lamoury FMJ, Caristo M et al (2011) Interferon-γ regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS ONE 6:e14698
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822
Bingisser RM, Tilbrook PA, Holt PG et al (1998) Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol 160:5729–5734
Glennie S, Soeiro I, Dyson PJ et al (2005) Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105:2821–2827
Benvenuto F, Ferrari S, Gerdoni E et al (2007) Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells 25:1753–1760
Nasef A, Mathieu N, Chapel A et al (2007) Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 84:231–237
Carosella ED, Moreau P, Le Maoult J et al (2003) HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol 81:199–252
Ryan JM, Barry F, Murphy JM et al (2007) Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol 149:353–363
English K, Barry FP, Field-Corbett CP et al (2007) IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett 110:91–100
Beyth S, Borovsky Z, Mevorach D et al (2005) Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105:2214–2219
Chabannes D, Hill M, Merieau E et al (2007) A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 110:3691–3694
González MA, Gonzalez-Rey E, Rico L et al (2009) Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 60:1006–1019
Caruso R, Marafini I, Sedda S et al (2014) Analysis of the cytokine profile in the duodenal mucosa of refractory coeliac disease patients. Clin Sci 126:451–458
Jones S, Horwood N, Cope A et al (2007) The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol 179:2824–2831
Ren G, Su J, Zhang L et al (2009) Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 27:1954–1962
Honczarenko M, Le Y, Swierkowski M et al (2006) Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24:1030–1041
Ji JF, He BP, Dheen ST et al (2004) Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 22:415–427
Troncone R, Discepolo V (2014) Celiac disease and autoimmunity. J Pediatr Gastroenterol Nutr 59:S9–S11
Tang K, Xiao X, Liu D et al (2014) Autografting of bone marrow mesenchymal stem cells alleviates streptozotocin-induced diabetes in miniature pigs: real-time tracing with MRI in vivo. Int J Mol Med 33:1469–1476
Lee RH, Seo MJ, Reger RL et al (2006) Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA 103:17438–17443
Madec AM, Mallone R, Afonso G et al (2009) Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 52:1391–1399
Fiorina P, Jurewicz M, Augello A et al (2009) Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 183:993–1004
Pabst O, Mowat AM (2012) Oral tolerance to food protein. Mucosal Immunol 5:232–239
Augello A, Tasso R, Negrini SM et al (2007) Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum 56:1175–1186
Maccario R, Podestà M, Moretta A et al (2005) Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory-suppressive phenotype. Haematologica 90:516–525
Di Ianni M, Del Papa B, De Ioanni M et al (2008) Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 36:309–318
Prevosto C, Zancolli M, Carnevali P et al (2007) Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 92:881–888
Ciccocioppo R, Bernardo ME, Sgarella A et al (2011) Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 60:788–798
Sundin M, D’arcy P, Johansson CC et al (2011) Multipotent mesenchymal stromal cells express FoxP3: a marker for the immunosuppressive capacity? J Immunother 34:336–342
Ichii M, Oritani K, Yokota T et al (2008) Regulation of human B lymphopoiesis by the transforming growth factor-beta superfamily in a newly established coculture system using mesenchymal stem cells as a supportive microenvironment. Exp Hematol 36:587–597
Corcione A, Benvenuto F, Ferretti E et al (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107:367–372
Tabera S, Perez-Simon JA, Diez-Campelo M et al (2008) The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica 93:1301–1309
Rasmusson I, Le Blanc K, Sundberg B et al (2007) Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol 65:336–343
Traggiai E, Volpi S, Schena F et al (2008) Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematous patients. Stem Cells 26:562–569
Schena F, Gambini C, Gregorio A et al (2010) Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum 62:2776–2786
Yu J, Zheng C, Ren X et al (2010) Intravenous administration of bone marrow mesenchymal stem cells benefits experimental autoimmune myasthenia gravis mice through an immunomodulatory action. Scand J Immunol 72:242–249
Rafei M, Hsieh J, Fortier S et al (2008) Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood 112:4991–4998
Singh UP, Singh NP, Singh B et al (2011) Stem cells as potential therapeutic targets for inflammatory bowel disease. Front Biosci 2:993–1008
Lee RH, Pulin AA, Seo MJ et al (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5:54–63
Hee Yoo K, Keun Jang I, Woo Lee M et al (2009) Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 259:150–156
Lee OK, Kuo TK, Chen WM et al (2004) Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103:1669–1675
Sarugaser R, Lickorish D, Baksh D et al (2005) Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells 23:220–229
Tsai MS, Lee JL, Chang YJ et al (2004) Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 19:1450–1456
Yamahara K, Harada K, Ohshima M et al (2014) Comparison of angiogenic, cytoprotective, and immunosuppressive properties of human amnion- and chorion-derived mesenchymal stem cells. PLoS ONE 9(2):e88319. doi:10.1371/journal.pone.0088319
Parolini O, Alviano F, Bagnara GP et al (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 26:300–311
Barlow S, Brooke G, Chatterjee K et al (2008) Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev 17:1095–1107
Du MR, Guo PF, Piao HL et al (2014) Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal–fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol 192:1502–1511
Acknowledgments
This work was supported by the Grant of the European Regional Development Fund—Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123).
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ciccocioppo, R., Cangemi, G.C., Roselli, E.A. et al. Are stem cells a potential therapeutic tool in coeliac disease?. Cell. Mol. Life Sci. 72, 1317–1329 (2015). https://doi.org/10.1007/s00018-014-1797-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1797-7