Abstract
Research on the nanoscale membrane structures known as lipid rafts is relevant to the fields of cancer biology, inflammation and ischaemia. Lipid rafts recruit molecules critical to signalling and regulation of the invasion process in malignant cells, the leukocytes that provide immunity in inflammation and the endothelial cells that build blood and lymphatic vessels, as well as the patterning of neural networks. As angiogenesis is a common denominator, regulation of receptors and signalling molecules critical to angiogenesis is central to the design of new approaches aimed at reducing, promoting or normalizing the angiogenic process. The goal of this review is to highlight some of the key issues that indicate the involvement of endothelial cell lipid rafts at each step of so-called ‘sprouting angiogenesis’, from stimulation of the vascular endothelial growth factor to the choice of tip cells, activation of migratory and invasion pathways, recruitment of molecules that guide axons in vascular patterning and maturation of blood vessels. Finally, the review addresses opportunities for future studies to define how these lipid domains (and their constituents) may be manipulated to stimulate the so-called ‘normalization’ of vascular networks within tumors, and be identified as the main target, enabling the development of more efficient chemotherapeutics and cancer immunotherapies.




Similar content being viewed by others
Abbreviations
- ECs:
-
Endothelial cells
- EPCs:
-
Endothelial progenitor cells
- ECM:
-
Extracellular matrix
- VEGF:
-
Vascular endothelial growth factor
- LRs:
-
Lipid rafts
- GPI:
-
Glycosylphosphatidylinositol
- ssBLMs:
-
Solid-supported bilayer lipid membranes
- Cav-1:
-
Caveolin-1
- Cav:
-
Caveolin
- PTRF:
-
Polymerase I and transcript release factor
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- KDR:
-
Kinase insert domain receptor
- FLK1:
-
Fetal liver kinase 1
- VEGFR1/Flt1:
-
Vascular endothelial growth factor receptor 1/fms-related tyrosine kinase 1
- Prxs:
-
Peroxirederoxins
- ROS:
-
Reactive oxygen species
- MMP:
-
Metalloprotease
- TACE:
-
Tumor necrosis alpha converting enzyme
- MMPs:
-
Matrix metalloproteinases
- uPAR:
-
Urokinase-type-plasminogen activator receptor
- MT1-MMP:
-
Membrane-type-1-MMP
- DEP1:
-
Density-enhanced tyrosine phosphatase
- ERK1/2:
-
Extracellular signal-regulated kinase 1/2
- Robos:
-
Roundabouts
- UNC5B:
-
Unc-5 homolog B
- Nrps:
-
Neuropilins
- Slit1-3:
-
Slit homolog 1-3
- RGMa:
-
Repulsive guidance molecule a
- FLRT3:
-
Fibronectin and leucine rich transmembrane protein 3
- Semas:
-
Semaphorins
- vSMC:
-
Vascular smooth muscle cell
- PDGFRβ:
-
Platelet-derived growth factor receptor β
- S1P:
-
Sphingosine-1-phosphate
- TGF β1:
-
Transforming growth factor-β1 (TGFβ1)
- ALK1:
-
Activin receptor-like kinase 1
- ALK5:
-
Activin receptor-like kinase 5
- TIE2:
-
TEK tyrosine kinase
- Ang:
-
Angiopoietin
- n-3 PUFA:
-
n-3 polyunsaturated fatty acids
References
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660
Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8:464–478
Roura S, Gálvez-Montón C, Bayes-Genis A (2013) The challenges for cardiac vascular precursor cell therapy: lessons from a very elusive precursor. J Vasc Res 50:304–323
Logsdon EA, Finley SD, Popel AS et al (2014) A systems biology view of blood vessel growth and remodelling. J Cell Mol Med. doi:10.1111/jcmm.12164
Gerhardt H, Betsholtz C (2005) How do endothelial cells orientate? Experientia 94:3–15
Klagsbrun M, Eichmann A (2005) A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev 16:535–548
Ushio-Fukai M (2007) VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 9:731–739
Montuori N, Ragno P (2014) Role of uPA/uPAR in the modulation of angiogenesis. Chem Immunol Allergy 99:105–155
Wang Y, Nakayama M, Pitulescu ME et al (2010) Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465:483–486
Benedito R, Rocha SF, Woeste M et al (2012) Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature 484:110–114
Urbinati C, Ravelli C, Tanghetti E et al (2012) Substrate-immobilized HIV-1 Tat drives VEGFR2/α(v)β(3)-integrin complex formation and polarization in endothelial cells. Arterioscler Thromb Vasc Biol 32:e25–e34
Deb M, Sengupta D, Patra SK (2012) Integrin-epigenetics: a system with imperative impact on cancer. Cancer Metastasis Rev 31:221–234
Gentil-dit-Maurin A, Oun S, Almagro S et al (2010) Unraveling the distinct distributions of VE- and N-cadherins in endothelial cells: a key role for p120-catenin. Exp Cell Res 316:2587–2599
Pilarczyk M, Mateuszuk L, Rygula A et al (2014) Endothelium in spots: high-content imaging of lipid rafts clusters in db/db mice. PLoS ONE 9:e106065
Brown DA, London E (1998) Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 14:111–136
Anderson RG, Jacobson K (2002) A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296:1821–1825
Callera GE, Montezano AC, Yogi A, Tostes RC, Touyz RM (2007) Vascular signaling through cholesterol-rich domains: implications in hypertension. Curr Opin Nephrol Hypertens 16:90–104
Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1:31–39
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Kenworthy AK (2008) Lipid rafts, cholesterol, and the brain. Neuropharmacology 55:1265–1273
Pike LJ (2008) The challenge of lipid rafts. J Lipid Res 50:S323
Simons K, Ehehalt R (2002) Cholesterol, lipid rafts, and disease. J Clin Invest 110:597–603
Carver LA, Schnitzer JE, Anderson RG et al (2003) Role of caveolae and lipid rafts in cancer: workshop summary and future needs. Cancer Res 63(20):6571–6574
Jacobson K, Dietrich C (1999) Looking at lipid rafts? Trends Cell Biol 9:87–91
Simons K, Gerl MJ (2010) Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 11:688–699
Pike LJ (2006) Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J Lipir Res 47:1597–1598
Leslie M (2011) Mysteries of the cell. Do lipid rafts exist? Science 334:1046–1047
Frisz JF, Klitzing HA, Lou K et al (2013) Sphingolipid domains in the plasma membranes of fibroblasts are not enriched with cholesterol. J Biol Chem 288:16855–16861
Edwards PR, Lowe PA, Leatherbarrow RJ (1997) Ligand loading at the surface of an optical biosensor and its effect upon the kinetics of protein–protein interactions. J Mol Recognit 10:128–134
Mao Y, Tero R, Imai Y et al (2008) The morphology of GM1x/SM0.6–x/Chol0.4 planar bilayers supported on SiO2 surfaces. Chem Phys Lett 460:289–297
Margheri G, D’Agostino R, Trigari S et al (2014) The β-subunit of cholera toxin has a high affinity for ganglioside GM1 embedded into solidsupported lipid membranes with a lipid raft-like composition. Lipids 49:203–206
Margheri G, D’Agostino R, Del Rosso M et al (2013) Fabrication of GM3-enriched sphingomyelin/cholesterol solid-supported lipid membranes on Au/SiO2 plasmoni substrates. Lipids 48:739–747
Margheri G, D’Agostino R, Becucci L et al (2012) Surface plasmon resonance as detection tool for lipids lateral mobility in biomimetic membranes. Biomed Opt Express 3:3119–3126
Sotgia F, Martinez-Outschoorn UE, Howell A et al (2012) Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol Mech Dis 7:423–467
Fernandez I, Ying Y, Albanesi J et al (2002) Mechanisms of caveolin filament assembly. Proc Natl Acad Sci USA 99:11193–11198
Palade GE (1953) Fine structure of blood capillaries. J Appl Phys 24:1424 (Abstract)
Simionescu M, Simionescu N, Palade E (1974) Morphometric data on the endothelium of blood capillaries. J Cell Biol 60:128–152
Stan RV (2002) Structure and function of endothelial caveolae. Microsc Res Tech 57:350–364
Liu L, Pilch PF (2008) A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem 293:4314–4322
Aboulaich N, Vainonen JP, Stralfors P et al (2004) Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem J 383:237–248
Chadda R, Mayor S (2008) PTRF triggers a cave in. Cell 132:23–24
Schulte T, Paschke KA, Laessing U et al (1997) Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development 124:577–587
Solis GP, Hoegg M, Munderloh C et al (2007) Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J 403:313–322
Palade GE (1961) Blood capillaries of the heart and other organs. Circulation 24:368–388
Lucero HA, Robbins PW (2004) Lipid rafts–protein association and the regulation of protein activity. Arch Biochem Biophys 426:208–224
Sebastiao AM, Colino-Oliveira M, Assaife-Lopez A et al (2013) Lipid rafts, synaptic transmission and plasticity: impact in age-related neurodegenerative diseases. Neuropharmacology 64:97–107
Gonnord B, Blouin CM, Lamaze C (2012) Membrane trafficking and signalling: two sides of the same coin. Semin Cell Dev Biol 23:154–164
Sowa G (2012) Caveolae, cavins, and endothelial cell function: new insights. Front Physiol 2:120
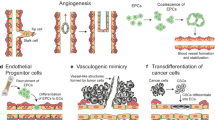
Cao Z, Shang B, Zhang G et al (2013) Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochim Byophys Acta 1836:273–286
Hendrix MJ, Seftor EA, Hess AR et al (2003) Vasculogenic mimicry and tumor-cell plasticity: lessons from melanoma. Nat Rev Cancer 3:411–421
Wang R, Chadalavada K, Wilshire J et al (2010) Glioblastoma stem-like cells give rise to tumor endothelium. Nature 468:829–833
Leenders WP, Kusters B, de Waal RM (2002) Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium 9:83–87
Kucera T, Lammert E (2009) Ancestral vascular tube formation and its adoption by tumors. Biol Chem 390:985–994
Gardner LB, Corn PG (2008) Hypoxic regulation of mRNA expression. Cell Cycle 7:1916–1924
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nature Med 9:669–676
Ladomery MR, Harper SJ, Bates DO (2006) Alternative splicing in angiogenesis: the vascular endothelial growth factor paradigm. Cancer Lett 249:133–142
Tan K, Jack Lawler J (2009) The interaction of Thrombospondins with extracellular matrix proteins. J Cell Commun Signal 3:177–187
Grant MA, Kalluri R (2005) Structural basis for the function of endogenous angiogenesis inhibitors. Cold Spring Harb Symp Quant Biol 70:399–410
Labrecque L, Royal I, Surprenant DS et al (2003) Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell 14:334–347
Kaverina I, Krylyshkina O, Small JV (2002) Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol 34:746–761
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436:193–200
Galli F, Piroddi M, Annetti C et al (2005) Oxidative stress and the reactive oxygen species. Contrib Nephrol 149:240–260
Shroder K (2010) Isoform specific functions of Nox protein-derived reactive oxygen species in the vasculature. Curr Opin Pharmacol 10:122–126
Oshikawa J, Urao N, Won Kim H et al (2010) Extracellular SOD-derived H2O2 promotes VEGF signalling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS ONE 5(4):e10189
Rhee GS, Kang SW, Jeong W et al (2005) Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17:183–189
Abid MR, Spokes KC, Shish SC et al (2007) NADPH oxidase activity selectively modulates vascular endothelial growth factor signalling pathways. J Biol Chem 282:35373–35385
Moldovan L, Mythreye K, GoldSchmidt-Clermont PJ et al (2006) Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc Res 71:236–246
Kang DH, LeeDJ Lee KW et al (2011) Peroxiredoxin II is an essential anti-oxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Mol Cell 44:545–558
Chillà A, Magherini F, Margheri F et al (2013) Proteomic identification of VEGF-dependent protein enrichment to membrane caveolar-raft microdomains in endothelial progenitor cells. Mol Cell Proteomics 12:1926–1938
Gorge KS, Wu S (2012) Lipid-raft: a floating island of death and survival. Toxicol Appl Pharmacol 259:311–319
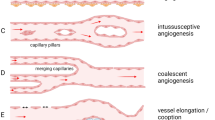
De Spiegelaere W, Casteleyn C, Van den Broeck W et al (2012) Intussusceptive angiogenesis: a biologically relevant form of angiogenesis. J Vasc Res 49:390–404
Thurston G, Kitajewski J (2008) VEGF and delta-Notch: interacting signaling pathways in tumor angiogenesis. Br J Cancer 99:1204–1209
Phng LK, Gerhardt H (2009) Angiogenesis: a team effort coordinated by notch. Dev Cell 16:196–208
Hellstrom M, Phng LK, Hofmann JJ et al (2007) Dll4 signaling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445:776–780
Lobov IB, Renard RA, Papadopoulos N et al (2007) Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenesis sprouting. Proc Natl Acad Sci USA 104:3219–3224
Benedito R, Roca C, Sörensen I (2009) The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137:1124–1135
Oswald F, Täuber B, Dobner T et al (2001) p300 acts as a transcriptional coactivator for mammalian Notch-1. Mol Cell Biol 21:7761–7774
Brou C, Logeat F, Gupta N et al (2000) A novel proteolytic cleavage involved in notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 5:207–216
Vetrivel KS, Cheng H, Lin W et al (2004) Association of γ-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem 279:44945–44954
Vetrivel KS, Thinakara G (2010) Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim Biophys Acta 1801:860–867
Hu J, Popp R, Fromel T et al (2014) Muller glia cells regulate Notch signaling and retinal angiogenesis via the generation of 19,20-dihydroxydocosapentaenoic acid. J Exp Med 211:281–295
Sacilotto N, Monteiro R, Fritzsche M et al (2013) Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci USA 110:11893–11898
Siekmann AF, Lawson ND (2007) Notch signalling and the regulation of angiogenesis. Cell Adh Migr 1:104–106
Siekmann AF, Covassin L, Lawson ND (2008) Modulation of VEGF signalling output by the Notch pathway. BioEssays 30:303–313
Hayashi K, Madri JA, Yurchenko PD (1992) Endothelial cells interact with the core protein of basement membrane perlecan through β1 and β3 integrin: an adhesion modulated by glycosaminoglycan. J Cell Biol 119:945–959
Hallman R, Horn N, Segl M et al (2005) Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev 85:979–1000
Chang SH, Kanasaki K, Gocheva V et al (2009) VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res 69:4537–4544
Pepper MS (2001) Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21:1104–1117
Van Hinsbergh VWM, Engelse MA, Quax PHA (2006) Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol 26:716–728
Senger DR, Davis GE (2011) Angiogenesis. Cold Spring Harb Perspect Biol 3:a005090. doi:10.1101/cshperspect.a005090
Breuss JM, Uhrin P (2012) VEGF-initiated angiogenesis and the uPA/uPAR system. Cell Adhes Migr 6:535–540
Uhrin P, Breuss JM (2013) uPAR: a modulator of VEGF-induced angiogenesis. Cell Adhes Migr 7:23–26
Koziol A, Martín-Alonso M, Clemente C et al (2012) Site-specific cellular functions of MT1-MMP. Eur J Cell Biol 91:889–895
Chun TH, Sabeh F, Ota I et al (2004) MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol 167:757–767
Gálvez BG, Matías-Román S, Yáñez-Mó M et al (2004) Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell 15:678–687
Gálvez BG, Matías-Román S, Yáñez-Mó M et al (2002) ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol 159:509–521
Frittoli E, Palamidessi A, Disanza A et al (2011) Secretory and endo/exocytic trafficking in invadopodia formation: the MT1-MMP paradigm. Eur J Cell Biol 90:108–114
Sundberg C, Nagy JA, Brown LF et al (2001) Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol 158:1145–1160
Saunders WB, Bohnsack BL, Faske JB et al (2006) Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 175:179–191
Stratman AN, Saunders WB, Sacharidou A et al (2009) Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood 114:237–247
Bayless KJ, Davis GE (2004) Microtubule depolymerization rapidly collapses capillary tube networks in vitro and angiogenic vessels in vivo through the small GTPase Rho. J Biol Chem 279:11686–11695
Davis GE, Camarillo CW (1996) An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res 224:39–51
Bayless KJ, Davis GE (2002) The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci 115:1123–1136
Davis GE, Bayless KJ (2003) An integrin and Rho GTPase-dependent pinocytic vacuole mechanism controls capillary lumen formation in collagen and fibrin matrices. Microcirculation 10:27–44
Kamei M, Saunders WB, Bayless KJ et al (2006) Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442:453–456
Lubarsky B, Krasnow MA (2003) Tube morphogenesis: making and shaping biological tubes. Cell 112:19–28
Lijnen HR (2002) Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry 67:107–115
Høyer-Hansen G, Rønne E, Solberg H et al (1992) Urokinase plasminogen activator cleaves its cell surface receptor releasing the ligand-binding domain. J Biol Chem 267:18224–18229
Ploug M, Rønne E, Behrendt N et al (1991) Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem 266:1926–1933
Sitrin RG, Johnson DR, Pan PM et al (2004) Lipid raft compartmentalization of urokinase receptor signaling in human neutrophils. Am J Respir Cell Mol Biol 30:233–241
Cunningham O, Andolfo A, Santovito ML et al (2003) Dimerization controls the lipid raft partitioning of uPAR/CD87 and regulates its biological functions. EMBO J 22:5994–6003
Sahores M, Prinetti A, Chiabrando G et al (2008) uPA binding increases UPAR localization to lipid rafts and modifies the receptor microdomain composition. Biochim Biophys Acta 1778:250–259
Rao JS, Gujrati M, Chetty C (2013) Tumor-associated soluble uPAR-directed endothelial cell motility and tumor angiogenesis. Oncogenesis 2:e53
Raghu H, Sodadasu PK, Malla RR et al (2010) Localization of uPAR and MMP-9 in lipid rafts is critical for migration, invasion and angiogenesis in human breast cancer cells. BMC Cancer 10:647
Margheri F, Chillà A, Laurenzana A et al (2011) Endothelial progenitor cell-dependent angiogenesis requires localization of the full-length form of uPAR in caveolae. Blood 118:3743–3755
Margheri F, Papucci L, Schiavone N et al (2015) Differential uPAR recruitment in caveolar-lipid rafts by GM1 and GM3 gangliosides regulates Endothelial progenitor cells angiogenesis. J Cell Mol Med 19:113–123
Del Rosso M, Fibbi G, Pucci M et al (2002) Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin Exp Metastasis 19:193–207
Blasi F, Carmeliet P (2002) uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 3:932–943
Kugler MC, Wei Y, Chapman HA (2003) Urokinase receptor and integrin interactions. Curr Pharm Des 9:1565–1574
D’Alessio S, Blasi F (2009) The urokinase receptor as an entertainer of signal transduction. Front Biosci (Landmark Ed) 14:4575–4587
Blasi F, Sidenius N (2010) The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett 584:1923–1930
Sidenius N, Blasi F (2000) Domain 1 of the urokinase receptor (uPAR) is required for uPAR-mediated cell binding to vitronectin. FEBS Lett 470:40–46
Eden G, Archinti M, Furlan F et al (2011) The urokinase receptor interactome. Curr Pharm Des 17:1874–1889
Stoppelli MP, Tacchetti C, Cubellis MV et al (1986) Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell 45:675–684
Stephens RW, Pöllänen J, Tapiovaara H et al (1989) Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol 108(5):1987–1995
Prager GW, Breuss JM, Steurer S et al (2004) Vascular endothelial growth factor (VEGF) induces rapid prourokinase (pro-uPA) activation on the surface of endothelial cells. Blood 103:955–962
Bajou K, Noël A, Gerard RD et al (1998) Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med 4:923–928
Bajou K, Masson V, Gerard RD et al (2001) The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J Cell Biol 152:777–784
Brunner PM, Heier PC, Mihaly-Bison J et al (2011) Density enhanced phosphatase-1 down-regulates urokinase receptor surface expression in confluent endothelial cells. Blood 117:4154–4161
Del Rosso M (2011) uPAR in angiogenesis regulation. Blood 117:3941–3943
Poettler M, Unseld M, Mihaly-Bison J et al (2012) The urokinase receptor (CD87) represents a central mediator of growth factor-induced endothelial cell migration. Thromb Haemost 108:357–366
Alexander RA, Prager GW, Mihaly-Bison J et al (2012) VEGF-induced endothelial cell migration requires urokinase receptor (uPAR)-dependent integrin redistribution. Cardiovasc Res 94:125–135
Schmaier AH, Larusch G (2010) Factor XII: new life for an old protein. Thromb Haemost 104:915–918
Chauvet S, Burk K, Mann F (2013) Navigation rules for vessels and neurons: cooperative signaling between VEGF and neural guidance cues. Cell Mol Life Sci 70:1685–1703
Adams RH, Eichmann A (2010) Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2:a001875. doi:10.1101/cshperspect.a001875
Larrivee B, Freitas C, Suchting S et al (2009) Guidance of vascular development: lessons from the nervous system. Circ Res 104:428–441
Wang B, XiaoY Ding B-B et al (2003) Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 4:19–29
Yadav SS, Narayan G (2014) Role of ROBO4 signalling in developmental and pathological angiogenesis. Biomed Res Int (683025)
Brose K, Bland KS, Wang KH et al (1999) Slit proteins bind robo receptors and have an evolutionary conserved role in repulsive axon guidance. Cell 96:795–806
Jones CA, London NR, Chen H et al (2008) Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med 14:448–453
Sheldon H, Andre M, Legg JA et al (2009) Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J 23:513–522
Zhang B, Dietrich UM, Geng JG et al (2009) Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood 114:430–439
Kaur S, Samant GV, Pramanik K et al (2008) Silencing of directional migration in roundabout4 knockdown endothelial cells. BMC Cell Biol 9:61
Maisse C, Rossin A, Cahuzac N et al (2008) Lipid raft localization and palmitoylation: identification of two requirements for cell death induction by the tumor suppressors UNC5H. Exp Cell Res 314:2544–2552
Koch AW, Mathivet T, Larrivée B et al (2011) Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell 20:33–46
Jin J, Sison K, Li C et al (2012) Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151:384–399
Lu X, Le Noble F, Yuan L et al (2004) The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature 432:179–186
Larrivée B, Freitas C, Trombe M et al (2007) Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev 21:2433–2447
Lejmi E, Leconte L, Pédron-Mazoyer S et al (2008) Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc Natl Acad Sci USA 105:2491–2496
Tassew NG, Mothe AJ, Shabanzadeh AP et al (2014) Modifying lipid rafts promotes regeneration and functional recovery. Cell Rep 8:1146–1159
Karaulanov E, Böttcher RT, Stannek P et al (2009) Unc5B interacts with FLRT3 and Rnd1 to modulate cell adhesion in Xenopus embryos. PLoS ONE 4:e5742
Pasterkamp RJ, Kolodkin AL (2003) Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol 13:79–89
Gu C, Giraudo E (2013) The role of semaphorins and their receptors in vascular development and cancer. Exp Cell Res 319:1306–1316
Gu C, Yoshida Y, Livet JETAL (2006) Smaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 307:265–268
Appleton BA, Wu P, Maloney J et al (2007) Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J 26:4902–4912
Guirland C, Suzuki S, Kojima METAL (2004) Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron 42:51–62
Campbell DS, Holt CE (2003) Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron 37:939–952
Salikhova A, Wang L, Lanahan AA et al (2008) Vascular endothelial growth factor and semaphorin induce neuropilin-1 endocytosis via separate pathways. Circ Res 103:e71–e79
Carcea I, Ma’ayan A, Mesias R et al (2010) Flotillin-mediated endocytic events dictate cell type-specific responses to semaphorin 3A. J Neurosci 30:15317–15329
Chen H, Bagri A, Zupicich JA et al (2000) Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron 25:43–56
Pasquale EB (2003) Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 6:462–475
Ogawa K, Pasqualini R, Lindberg RA et al (2000) The ephrin-A1 ligand and its receptor EphA2, are expressed during tumor neovascularization. Oncogene 19:6043–6052
Brantley-Sieders DM, Fang WB, Hwang Y et al (2006) Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res 66:10315–10324
Foo SS, Turner CJ, Adams S et al (2006) Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124:161–173
Wang HU, Chen ZF, Anderson DJ (1998) Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93:741–753
Gale NW, Baluk P, Pan L et al (2001) Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol 230:151–160
Noren NK, Foos G, Hauser CA et al (2006) The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol 8:815–825
Erber R, Eichelsbacher U, Powajbo V et al (2006) EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J 25:628–641
Blits-Huizinga CT, Nelersa CM, Malhotra A et al (2004) Ephrins and their receptors: binding versus biology. IUBMB Life 56:257–265
Wimmer-Kleikamp SH, Janes PW, Squire A et al (2004) Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol 164:661–666
Gauthier LR, Robbins SM (2003) Ephrin signaling: One raft to rule them all? One raft to sort them? One raft to spread their call and in signaling bind them? Life Sci 74:207–216
Vearing CJ, Lackmann M (2005) Eph receptor signalling; dimerisation just isn’t enough. Growth Factors 23:67–76
Himanen JP, Saha N, Nikolov DB (2007) Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol 19:534–542
Wu C, Butz S, Ying Y et al (1997) Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J Biol Chem 272:3554–3559
Bocharov EV, Mayzel ML, Volynsky PE et al (2010) Left-handed dimer of EphA2 transmembrane domain: helix packing diversity among receptor tyrosine kinases. Biophys J 98:881–889
Ahn GO, Brown JM (2009) Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis 12:159–164
Psaltis PJ, Harbuzariu A, Delacroix S et al (2011) Resident vascular progenitor cells–diverse origins, phenotype, and function. J Cardiovasc Transl Res 4:161–176
Marcelo KL, Goldie LC, Hirschi KK (2013) Regulation of endothelial cell differentiation and specification. Circ Res 112:1272–1287
Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97:512–523
Betsholtz C, Lindblom P, Gerhardt H (2005) Role of pericytes in vascular morphogenesis. EXS 94:115–125
Stehr M, Adam RM, Khoury J et al (2003) Platelet derived growth factor-BB is a potent mitogen for rat ureteral and human bladder smooth muscle cells: dependence on lipid rafts for cell signaling. J Urol 169:1165–1170
Kiyan J, Haller H, Dumler I (2009) The tyrosine phosphatase SHP-2 controls urokinase-dependent signaling and functions in human vascular smooth muscle cells. Exp Cell Res 315:1029–1039
Kiyan J, Smith G, Haller H et al (2009) Urokinase-receptor-mediated phenotypic changes in vascular smooth muscle cells require the involvement of membrane rafts. Biochem J 423:343–351
Nakayama A, Nakayama M, Turner CJ et al (2013) Ephrin-B2 controls PDGFRβ internalization and signaling. Genes Dev 27:2576–2589
Spiegel S, Milstien S (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4:397–407
Kono M, Mi Y, Liu Y et al (2004) The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem 279:29367–29373
Oskouian B, Saba J (2007) Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle 6:522–527
Paik JH, Skoura A, Chae SS et al (2004) Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev 18:2392–2403
Kokudo T, Suzuki Y, Yoshimatsu Y et al (2008) Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci 121:3317–3324
Goumans MJ, Lebrin F, Valdimarsdottir G (2003) Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med 13:301–307
Itoh F, Itoh S, Adachi T et al (2012) Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. Blood 119:5320–5328
Chen YG (2009) Endocytic regulation of TGF-beta signaling. Cell Res 19:58–70
Thurston G (2003) Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res 314:61–68
Eklund L, Olsen BR (2006) Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res 312:630–641
Katoh SY, Kamimoto T, Yamakawa D et al (2009) Lipid rafts serve as signaling platforms for Tie2 receptor tyrosine kinase in vascular endothelial cells. Exp Cell Res 315:2818–2823
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407:249–257
Al-Husein B, Abdalla M, Trepte M et al (2012) Antiangiogenic therapy for cancer: an update. Pharmacotherapy 32:1095–1111
Grothey A, Allegra C (2012) Antiangiogenesis therapy in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol 4:301–319
Wu JM, Staton CA (2012) Anti-angiogenic drug discovery: lessons from the past and thoughts for the future. Expert Opin Drug Discov 7:723–743
Osinsky S, Zavelevich M, Vaupel P (2009) Tumor hypoxia and malignant progression. Exp Oncol 31:80–86
Kong DS, Song SY, Kim DH et al (2009) Prognostic significance of c-Met expression in glioblastomas. Cancer 115:140–148
Takeda T, Okuyama H, Nishizaka Y, Tomita S, Inoue M (2012) Hypoxia-inducible factor-1alpha is necessary for invasive phenotype in VEGF-deleted islet cell tumors. Sci Rep 2:494
Deryugina EI, Quigley JP (2010) Pleiotropic roles of matrox metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochim Biophys Acta 1803:103–120
Lu KV, Bergers G (2013) Mechanisms of evasive resistance to anti-VEGF therapy in glioblastoma. CNS Oncol 2:49–65
Lester RD, Jo M, Montel V, Takimoto S, Gonias SL (2007) uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol 30(178):425–436
Ellis LM, Hicklin DJ (2008) Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res 14:6371–6375
Casanovas O, Hicklin DJ, Bergers G, Hanahan D (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8:299–309
Yan M, Plowman GD (2007) Delta-like 4/Notch signaling and its therapeutic implications. Clin Cancer Res 13:7243–7246
Batchelor TT, Sorensen AG, di Tomaso E et al (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Fischer C, Jonckx B, Mazzone M et al (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131:463–475
Winkler F, Kozin SV, Tong RT et al (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6:553–563
Shojaei F, Wu X, Malik AK et al (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+ Gr1+ myeloid cells. Nat Biotechnol 25:911–920
Carmeliet P, Jain RK (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 10:417–427
Shang B, Cao Z, Zhou Q (2012) Progress in tumor vascular normalization for anticancer therapy: challenge and perspectives. Front Med 6:67–78
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 2011(473):298–307
Trojanowska M (2010) Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat Rev Rheumatol 6:453–460
Murdaca G, Colombo BM, Cagnati P, Gulli R, Spanò F, Puppo F (2012) Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 224:309–317
Hannex BH (2013) Therapeutic angiogenesis for critical limb ischemia. Nat Rev Cardiol 10:387–396
Zhang H, van Olden C, Sweeney D, Martin-Rendon E (2014) Blood vessel repair and regeneration in the ischaemic heart. Open Heart 1:e000016
Van de Veire S, Stalmans I, Heindryckx F et al (2010) Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell 141:178–190
Van Steenkiste C, Geerts A, Vanheule E et al (2009) Role of placental growth factor in mesenteric neoangiogenesis in a mouse model of portal hypertension. Gastroenterology 137:2112–2124
Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd et al (2009) Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 361:358–367
Williams TM, Lisanti MP (2004) The caveolin genes: from cell biology to medicine. Ann Med 36:584–595
Navarro G, Borroto-Escuela DO, Fuxe K, Franco R (2014) Potential of caveolae in the therapy of cardiovascular and neurological diseases. Front Physiol 5:370
Fridolfsson HN, Patel HH (2013) Caveolin and caveolae in age-associated cardiovascular disease. J Geriatr Cardiol 10:66–74
Galbiati F, Engelman JA, Volonte D et al (2001) Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem 276(24):21425–21433
Roura S, Cal R, Gálvez-Montón C et al (2014) Inverse relationship between raft LRP1 localization and non-raft ERK1,2/MMP9 activation in idiopathic dilated cardiomyopathy: potential impact in ventricular remodeling. Int J Cardiol 176:805–814
Gumbleton M, Hollins AJ, Omidi Y, Campbell L, Taylor G (2003) Targeting caveolae for vesicular drug transport. J Control Release 87:139–151
Chapkin RS, Wang N, Fan Y-Y et al (2008) Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochem Biophys Acta 1778:466–471
Szymczak M, Murray M, Petrovic N (2008) Modulation of angiogenesis by omega-3 polyunsaturated fatty acids is mediated by cyclooxygenases. Blood 111:3514–3521
Wang J, Shi Y, Zhang L et al (2014) Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis 68:91–103
Connor KM, SanGiovanni JP, Lofqvist C et al (2007) Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med 13:868–873
Acknowledgments
This work was supported by grants of the Ente Cassa di Risparmio di Firenze to MDR, GF and GM; Istituto Toscano Tumori to MDR; Associazione Italiana Ricerca sul Cancro to MDR (AIRC Grant No. IG 2013 N. 14266); Brazilian FAPERJ to TDR. FM was supported by a post-doctoral joint fellowship of the European Union and Regione Toscana within the project UNIFI-4 MELOTAC.
Author information
Authors and Affiliations
Corresponding authors
Additional information
A. Laurenzana and G. Fibbi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Laurenzana, A., Fibbi, G., Chillà, A. et al. Lipid rafts: integrated platforms for vascular organization offering therapeutic opportunities. Cell. Mol. Life Sci. 72, 1537–1557 (2015). https://doi.org/10.1007/s00018-014-1814-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1814-x