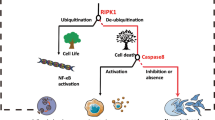
Abstract
Regulated cell death is one major factor to ensure homoeostasis in multicellular organisms. For decades, apoptosis was considered as the sole form of regulated cell death, whereas necrosis was believed to be accidental and unregulated. Due to this view, research on necrosis was somewhat neglected, especially in the field of anti-cancer treatment. However, new interest in necrosis has been sparked by the recent discovery of different forms of necrosis that show indeed regulated pathways. More and more studies now address the molecular pathways of regulated necrosis and its connections within the cellular signaling networks. Necroptosis, a subform of regulated necrosis, has so far hardly been focused on with regard to a future treatment of cancer patients and may emerge as a novel and effective approach to eliminate tumor cells. However, and similar to apoptosis, tumor cells can develop resistances against necroptosis to ensure their own survival. In this context, new molecules that enhance necroptosis are currently being identified to overcome such resistances. This review discusses cancer and necroptosis as friends or foes, i.e. the options to exploit necroptosis in anti-cancer therapies (“foes”), but also potential limitations that may block or actually cause necroptosis to act in a protumoral manner (“friends”). The balance between these two possible roles will determine whether necroptosis can indeed be used as a promising tool for early diagnosis of tumors, prevention of metastasis and anti-cancer treatment.


Similar content being viewed by others
References
Lockshin RA, Zakeri Z (2007) Cell death in health and disease. J Cell Mol Med 11(6):1214–1224. doi:10.1111/j.1582-4934.2007.00150.x
Lockshin RA, Williams CM (1964) Programmed cell death. 2. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J Insect Physiol 10(4):643–649. doi:10.1016/0022-1910(64)90034-4
Ellis HM, Horvitz HR (1986) Genetic-control of programmed cell-death in the nematode C. elegans. Cell 44(6):817–829. doi:10.1016/0092-8674(86)90004-8
Golstein P, Kroemer G (2007) Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 32(1):37–43. doi:10.1016/j.tibs.2006.11.001
Laster SM, Wood JG, Gooding LR (1988) Tumor necrosis factor can induce both apoptic and necrotic forms of cell-lysis. J Immunol 141(8):2629–2634
Vercammen D, Vandenabeele P, Beyaert R, Declercq W, Fiers W (1997) Tumour necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine 9(11):801–808. doi:10.1006/cyto.1997.0252
Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S (1998) Caspase-independent cell killing by Fas-associated protein with death domain. J Cell Biol 143(5):1353–1360. doi:10.1083/jcb.143.5.1353
Ziegler U, Groscurth P (2004) Morphological features of cell death. News Physiol Sci 19:124–128. doi:10.1152/nips.01519.2004
Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Bio 15(2):134–146. doi:10.1038/nrm3737
Christofferson DE, Yuan JY (2010) Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22(2):263–268. doi:10.1016/j.ceb.2009.12.003
Sun LM, Wang HY, Wang ZG, He SD, Chen S, Liao DH, Wang L, Yan JC, Liu WL, Lei XG, Wang XD (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148(1–2):213–227. doi:10.1016/j.cell.2011.11.031
Fuchs Y, Steller H (2015) Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Bio 16(6):329–344. doi:10.1038/nrm3999
Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517(7534):311–320. doi:10.1038/nature14191
Takemura R, Takaki H, Okada S, Shime H, Akazawa T, Oshiumi H, Matsumoto M, Teshima T, Seya T (2015) PolyI:C-induced, TLR3/RIP3-dependent necroptosis backs up immune effector-mediated tumor elimination in vivo. Cancer Immunol Res 3(8):902–914. doi:10.1158/2326-6066.CIR-14-0219
Schmidt SV, Seibert S, Walch-Ruckheim B, Vicinus B, Kamionka EM, Pahne-Zeppenfeld J, Solomayer EF, Kim YJ, Bohle RM, Smola S (2015) RIPK3 expression in cervical cancer cells is required for PolyIC-induced necroptosis, IL-1 alpha release, and efficient paracrine dendritic cell activation. Oncotarget 6(11):8635–8647
Kaczmarek A, Vandenabeele P, Krysko DV (2013) Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38(2):209–223. doi:10.1016/j.immuni.2013.02.003
Linkermann A, Green DR (2014) Necroptosis. New Engl J Med 370(5):455–465. doi:10.1056/Nejmra1310050
Inoue H, Tani K (2014) Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ 21(1):39–49. doi:10.1038/cdd.2013.84
Guo ZS, Liu Z, Bartlett DL (2014) Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol 4:74. doi:10.3389/fonc.2014.00074
Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, Green DR, Oberst A, Albert ML (2015) RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science 350(6258):328–334. doi:10.1126/science.aad0395
Fulda S (2014) Therapeutic exploitation of necroptosis for cancer therapy. Semin Cell Dev Biol 35:51–56. doi:10.1016/j.semcdb.2014.07.002
Dunai ZA, Imre G, Barna G, Korcsmaros T, Petak I, Bauer PI, Mihalik R (2012) Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937 cells. PLoS One 7(7):e41945. doi:10.1371/journal.pone.0041945
McCabe KE, Bacos K, Lu D, Delaney JR, Axelrod J, Potter MD, Vamos M, Wong V, Cosford ND, Xiang R, Stupack DG (2014) Triggering necroptosis in cisplatin and IAP antagonist-resistant ovarian carcinoma. Cell Death Dis 5:e1496. doi:10.1038/cddis.2014.448
Voigt S, Philipp S, Davarnia P, Winoto-Morbach S, Röder C, Arenz C, Trauzold A, Kabelitz D, Schütze S, Kalthoff H, Adam D (2014) TRAIL-induced programmed necrosis as a novel approach to eliminate tumor cells. BMC Cancer 14:74. doi:10.1186/1471-2407-14-74
Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 187(9):1477–1485. doi:10.1084/jem.187.9.1477
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1(6):489–495. doi:10.1038/82732
Callus BA, Vaux DL (2007) Caspase inhibitors: viral, cellular and chemical. Cell Death Differ 14(1):73–78. doi:10.1038/sj.cdd.4402034
Fulda S, Vucic D (2012) Targeting IAP proteins for therapeutic intervention in cancer. Nature Rev Drug Discov 11(2):109–124. doi:10.1038/nrd3627
Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK (2015) Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis 6:e1636. doi:10.1038/cddis.2015.16
He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137(6):1100–1111. doi:10.1016/j.cell.2009.05.021
Steinhart L, Belz K, Fulda S (2013) Smac mimetic and demethylating agents synergistically trigger cell death in acute myeloid leukemia cells and overcome apoptosis resistance by inducing necroptosis. Cell Death Dis 4:e802. doi:10.1038/cddis.2013.320
Grassilli E, Ianzano L, Bonomo S, Missaglia C, Cerrito MG, Giovannoni R, Masiero L, Lavitrano M (2014) GSK3A is redundant with GSK3B in modulating drug resistance and chemotherapy-induced necroptosis. PLoS One 9(7):e100947. doi:10.1371/journal.pone.0100947
Okada M, Adachi S, Imai T, Watanabe K, Toyokuni SY, Ueno M, Zervos AS, Kroemer G, Nakahata T (2004) A novel mechanism for imatinib mesylate-induced cell death of BCR-ABL-positive human leukemic cells: caspase-independent, necrosis-like programmed cell death mediated by serine protease activity. Blood 103(6):2299–2307. doi:10.1182/blood-2003-05-1605
Xu Y, Lin Z, Zhao N, Zhou L, Liu F, Cichacz Z, Zhang L, Zhan Q, Zhao X (2014) Receptor interactive protein kinase 3 promotes cisplatin-triggered necrosis in apoptosis-resistant esophageal squamous cell carcinoma cells. PLoS One 9(6):e100127. doi:10.1371/journal.pone.0100127
Huang CJ, Luo YA, Zhao JW, Yang FW, Zhao HW, Fan WH, Ge PF (2013) Shikonin kills glioma cells through necroptosis mediated by RIP-1. PLoS One 8(6):e66326. doi:10.1371/journal.pone.0066326
Wada N, Kawano Y, Fujiwara S, Kikukawa Y, Okuno Y, Tasaki M, Ueda M, Ando Y, Yoshinaga K, Ri M, Iida S, Nakashima T, Shiotsu Y, Mitsuya H, Hata H (2015) Shikonin, dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells. Int J Oncol 46(3):963–972. doi:10.3892/ijo.2014.2804
Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X (2011) Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 30(42):4297–4306. doi:10.1038/onc.2011.137
Yu X, Deng Q, Li W, Xiao L, Luo X, Liu X, Yang L, Peng S, Ding Z, Feng T, Zhou J, Fan J, Bode AM, Dong Z, Liu J, Cao Y (2015) Neoalbaconol induces cell death through necroptosis by regulating RIPK-dependent autocrine TNFalpha and ROS production. Oncotarget 6(4):1995–2008
Deng Q, Yu X, Xiao L, Hu Z, Luo X, Tao Y, Yang L, Liu X, Chen H, Ding Z, Feng T, Tang Y, Weng X, Gao J, Yi W, Bode AM, Dong Z, Liu J, Cao Y (2013) Neoalbaconol induces energy depletion and multiple cell death in cancer cells by targeting PDK1-PI3-K/Akt signaling pathway. Cell Death Dis 4:e804. doi:10.1038/cddis.2013.324
Pasupuleti N, Leon L, Carraway KL 3rd, Gorin F (2013) 5-Benzylglycinyl-amiloride kills proliferating and nonproliferating malignant glioma cells through caspase-independent necroptosis mediated by apoptosis-inducing factor. J Pharmacol Exp Ther 344(3):600–615. doi:10.1124/jpet.112.200519
Babcook MA, Sramkoski RM, Fujioka H, Daneshgari F, Almasan A, Shukla S, Nanavaty RR, Gupta S (2014) Combination simvastatin and metformin induces G1-phase cell cycle arrest and Ripk1- and Ripk3-dependent necrosis in C4-2B osseous metastatic castration-resistant prostate cancer cells. Cell Death Dis 5:e1536. doi:10.1038/cddis.2014.500
Hernandez-Breijo B, Monserrat J, Ramirez-Rubio S, Cuevas EP, Vara D, Diaz-Laviada I, Fernandez-Moreno MD, Roman ID, Gisbert JP, Guijarro LG (2011) Preclinical evaluation of azathioprine plus buthionine sulfoximine in the treatment of human hepatocarcinoma and colon carcinoma. World J Gastroenterol 17(34):3899–3911. doi:10.3748/wjg.v17.i34.3899
Coupienne I, Bontems S, Dewaele M, Rubio N, Habraken Y, Fulda S, Agostinis P, Piette J (2011) NF-kappaB inhibition improves the sensitivity of human glioblastoma cells to 5-aminolevulinic acid-based photodynamic therapy. Biochem Pharmacol 81(5):606–616. doi:10.1016/j.bcp.2010.12.015
Rizzi F, Naponelli V, Silva A, Modernelli A, Ramazzina I, Bonacini M, Tardito S, Gatti R, Uggeri J, Bettuzzi S (2014) Polyphenon E(R), a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis 35(4):828–839. doi:10.1093/carcin/bgt481
Wallenberg M, Misra S, Wasik AM, Marzano C, Björnstedt M, Gandin V, Fernandes AP (2014) Selenium induces a multi-targeted cell death process in addition to ROS formation. J Cell Mol Med 18(4):671–684. doi:10.1111/jcmm.12214
Liu X, Chhipa RR, Nakano I, Dasgupta B (2014) The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol Cancer Ther 13(3):596–605. doi:10.1158/1535-7163.MCT-13-0579
Deeraksa A, Pan J, Sha Y, Liu XD, Eissa NT, Lin SH, Yu-Lee LY (2013) Plk1 is upregulated in androgen-insensitive prostate cancer cells and its inhibition leads to necroptosis. Oncogene 32(24):2973–2983. doi:10.1038/onc.2012.309
Tufo G, Jones AW, Wang Z, Hamelin J, Tajeddine N, Esposti DD, Martel C, Boursier C, Gallerne C, Migdal C, Lemaire C, Szabadkai G, Lemoine A, Kroemer G, Brenner C (2014) The protein disulfide isomerases PDIA4 and PDIA6 mediate resistance to cisplatin-induced cell death in lung adenocarcinoma. Cell Death Differ 21(5):685–695. doi:10.1038/cdd.2013.193
Thon L, Mathieu S, Kabelitz D, Adam D (2006) The murine TRAIL receptor signals caspase-independent cell death through ceramide. Exp Cell Res 312(19):3808–3821. doi:10.1016/j.yexcr.2006.08.017
Thon L, Möhlig H, Mathieu S, Lange A, Bulanova E, Winoto-Morbach S, Schütze S, Bulfone-Paus S, Adam D (2005) Ceramide mediates caspase-independent programmed cell death. FASEB J 19(14):1945–1956. doi:10.1096/fj.05-3726com
Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, Bielawski J, Szulc ZM, Thomas RJ, Selvam SP, Senkal CE, Garrett-Mayer E, De Palma RM, Fedarovich D, Liu A, Habib AA, Stahelin RV, Perrotti D, Ogretmen B (2013) Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med 5(1):105–121. doi:10.1002/emmm.201201283
Yamanaka K, Urano Y, Takabe W, Saito Y, Noguchi N (2014) Induction of apoptosis and necroptosis by 24(S)-hydroxycholesterol is dependent on activity of acyl-CoA:cholesterol acyltransferase 1. Cell Death Dis 5:e990. doi:10.1038/cddis.2013.524
Dai X, Zhang J, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Kumar AP, Ahn KS, Sethi G (2015) Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med 240(6):760–773. doi:10.1177/1535370215579167
Philipp S, Sosna J, Plenge J, Kalthoff H, Adam D (2015) Homoharringtonine, a clinically approved anti-leukemia drug, sensitizes tumor cells for TRAIL-induced necroptosis. Cell Commun Signal 13:25. doi:10.1186/s12964-015-0103-0
Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D, Vandenabeele P, Samson M, Dimanche-Boitrel MT (2012) TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ 19(12):2003–2014. doi:10.1038/cdd.2012.90
Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, Mocarski ES (2015) Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J Biol Chem 290(18):11635–11648. doi:10.1074/jbc.M115.646042
Irrinki KM, Mallilankaraman K, Thapa RJ, Chandramoorthy HC, Smith FJ, Jog NR, Gandhirajan RK, Kelsen SG, Houser SR, May MJ, Balachandran S, Madesh M (2011) Requirement of FADD, NEMO, and BAX/BAK for aberrant mitochondrial function in tumor necrosis factor alpha-induced necrosis. Mol Cell Biol 31(18):3745–3758. doi:10.1128/MCB.05303-11
Lamothe B, Lai Y, Xie M, Schneider MD, Darnay BG (2013) TAK1 is essential for osteoclast differentiation and is an important modulator of cell death by apoptosis and necroptosis. Mol Cell Biol 33(3):582–595. doi:10.1128/MCB.01225-12
O’Donnell MA, Hase H, Legarda D, Ting AT (2012) NEMO inhibits programmed necrosis in an NFkappaB-independent manner by restraining RIP1. PLoS ONE 7(7):e41238. doi:10.1371/journal.pone.0041238
Liu M, Gu XH, Zhang K, Ding Y, Wei XB, Zhang XM, Zhao YX (2013) Gold nanoparticles trigger apoptosis and necrosis in lung cancer cells with low intracellular glutathione. J Nanopart Res. doi:10.1007/S11051-013-1745-8
Akhtar MJ, Alhadlaq HA, Kumar S, Alrokayan SA, Ahamed M (2015) Selective cancer-killing ability of metal-based nanoparticles: implications for cancer therapy. Arch Toxicol. doi:10.1007/s00204-015-1570-1
van Marion DM, Domanska UM, Timmer-Bosscha H, Walenkamp AM (2016) Studying cancer metastasis: existing models, challenges and future perspectives. Crit Rev Oncol Hematol 97:107–117. doi:10.1016/j.critrevonc.2015.08.009
Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12(8):895–904. doi:10.1038/nm1469
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2(8):563–572. doi:10.1038/nrc865
Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC (1998) Multistep nature of metastatic inefficiency—dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153(3):865–873. doi:10.1016/S0002-9440(10)65628-3
Su Z, Yang Z, Xu Y, Chen Y, Yu Q (2015) Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer 14(1):48. doi:10.1186/s12943-015-0321-5
Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, Emme D, Röder C, Kalthoff H, Wajant H (2006) TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene 25(56):7434–7439. doi:10.1038/sj.onc.1209719
Röder C, Trauzold A, Kalthoff H (2011) Impact of death receptor signaling on the malignancy of pancreatic ductal adenocarcinoma. Eur J Cell Biol 90(6–7):450–455. doi:10.1016/j.ejcb.2010.10.008
Lemke J, von Karstedt S, Zinngrebe J, Walczak H (2014) Getting TRAIL back on track for cancer therapy. Cell Death Differ 21(9):1350–1364. doi:10.1038/cdd.2014.81
Haselmann V, Kurz A, Bertsch U, Hübner S, Olempska-Müller M, Fritsch J, Häsler R, Pickl A, Fritsche H, Annewanter F, Engler C, Fleig B, Bernt A, Röder C, Schmidt H, Gelhaus C, Hauser C, Egberts JH, Heneweer C, Rohde AM, Böger C, Knippschild U, Röcken C, Adam D, Walczak H, Schütze S, Janssen O, Wulczyn FG, Wajant H, Kalthoff H, Trauzold A (2014) Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 146(1):278–290. doi:10.1053/j.gastro.2013.10.009
Prasad S, Kim JH, Gupta SC, Aggarwal BB (2014) Targeting death receptors for TRAIL by agents designed by Mother Nature. Trends Pharmacol Sci 35(10):520–536. doi:10.1016/j.tips.2014.07.004
Fu ZZ, Deng BY, Liao YX, Shan LC, Yin F, Wang ZY, Zeng H, Zuo DQ, Hua YQ, Cai ZD (2013) The anti-tumor effect of shikonin on osteosarcoma by inducing RIP1 and RIP3 dependent necroptosis. BMC Cancer 13:580. doi:10.1186/1471-2407-13-580
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11(10):700–714. doi:10.1038/nrm2970
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325(5938):332–336. doi:10.1126/science.1172308
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123. doi:10.1016/j.cell.2009.05.037
Ryter SW, Mizumura K, Choi AM (2014) The impact of autophagy on cell death modalities. Int J Cell Biol 2014:502676. doi:10.1155/2014/502676
Parikh SA, Kantarjian H, Schimmer A, Walsh W, Asatiani E, El-Shami K, Winton E, Verstovsek S (2010) Phase II study of obatoclax mesylate (GX15-070), a small-molecule BCL-2 family antagonist, for patients with myelofibrosis. Clin Lymphoma Myeloma Leuk 10(4):285–289. doi:10.3816/CLML.2010.n.059
Basit F, Cristofanon S, Fulda S (2013) Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ 20(9):1161–1173. doi:10.1038/cdd.2013.45
Urtishak KA, Edwards AY, Wang LS, Hudome A, Robinson BW, Barrett JS, Cao K, Cory L, Moore JS, Bantly AD, Yu QC, Chen IM, Atlas SR, Willman CL, Kundu M, Carroll AJ, Heerema NA, Devidas M, Hilden JM, Dreyer ZE, Hunger SP, Reaman GH, Felix CA (2013) Potent obatoclax cytotoxicity and activation of triple death mode killing across infant acute lymphoblastic leukemia. Blood 121(14):2689–2703. doi:10.1182/blood-2012-04-425033
Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK, Schäfer BW, Schrappe M, Stanulla M, Bourquin JP (2010) Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest 120(4):1310–1323. doi:10.1172/JCI39987
He W, Wang Q, Srinivasan B, Xu J, Padilla MT, Li Z, Wang X, Liu Y, Gou X, Shen HM, Xing C, Lin Y (2014) A JNK-mediated autophagy pathway that triggers c-IAP degradation and necroptosis for anticancer chemotherapy. Oncogene 33(23):3004–3013. doi:10.1038/onc.2013.256
Colbert LE, Fisher SB, Hardy CW, Hall WA, Saka B, Shelton JW, Petrova AV, Warren MD, Pantazides BG, Gandhi K, Kowalski J, Kooby DA, El-Rayes BF, Staley CA 3rd, Adsay NV, Curran WJ Jr, Landry JC, Maithel SK, Yu DS (2013) Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer 119(17):3148–3155. doi:10.1002/cncr.28144
He L, Peng K, Liu Y, Xiong J, Zhu FF (2013) Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. OncoTargets Ther 6:1539–1543. doi:10.2147/OTT.S52805
Wang HY, Sun LM, Su LJ, Rizo J, Liu L, Wang LF, Wang FS, Wang XD (2014) Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54(1):133–146. doi:10.1016/j.molcel.2014.03.003
Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, Menzies A, Teague JW, Futreal PA, Stratton MR (2008) The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr Protoc Hum Genet Chapter 10:Unit 10 11. doi:10.1002/0471142905.hg1011s57
Linkermann A, Stockwell BR, Krautwald S, Anders HJ (2014) Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol 14(11):759–767. doi:10.1038/nri3743
Brenner D, Blaser H, Mak TW (2015) Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol 15(6):362–374. doi:10.1038/nri3834
Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M (2011) The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35(4):572–582. doi:10.1016/j.immuni.2011.08.014
Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M (2011) FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477(7364):330–334. doi:10.1038/nature10273
Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C (2011) Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 477(7364):335–339. doi:10.1038/nature10400
Pierdomenico M, Negroni A, Stronati L, Vitali R, Prete E, Bertin J, Gough PJ, Aloi M, Cucchiara S (2014) Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am J Gastroenterol 109(2):279–287. doi:10.1038/ajg.2013.403
Huang CY, Kuo WT, Huang YC, Lee TC, Yu LC (2013) Resistance to hypoxia-induced necroptosis is conferred by glycolytic pyruvate scavenging of mitochondrial superoxide in colorectal cancer cells. Cell Death Dis 4:e622. doi:10.1038/cddis.2013.149
Cerhan JR, Ansell SM, Fredericksen ZS, Kay NE, Liebow M, Call TG, Dogan A, Cunningham JM, Wang AH, Liu-Mares W, Macon WR, Jelinek D, Witzig TE, Habermann TM, Slager SL (2007) Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood 110(13):4455–4463. doi:10.1182/blood-2007-05-088682
Nugues AL, El Bouazzati H, Hetuin D, Berthon C, Loyens A, Bertrand E, Jouy N, Idziorek T, Quesnel B (2014) RIP3 is downregulated in human myeloid leukemia cells and modulates apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis 5:e1384. doi:10.1038/cddis.2014.347
Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, Koo JS, Kim SI, Kim SJ, Son MK, Hong SS, Levy JM, Pollyea DA, Jordan CT, Yan P, Frankhouser D, Nicolet D, Maharry K, Marcucci G, Choi KS, Cho H, Thorburn A, Kim YS (2015) Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res 25(6):707–725. doi:10.1038/cr.2015.56
Chen W, Wu J, Li L, Zhang Z, Ren J, Liang Y, Chen F, Yang C, Zhou Z, Su SS, Zheng X, Zhang Z, Zhong CQ, Wan H, Xiao M, Lin X, Feng XH, Han J (2015) Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat Cell Biol 17(4):434–444. doi:10.1038/ncb3120
Lu X, An H, Jin R, Zou M, Guo Y, Su PF, Liu D, Shyr Y, Yarbrough WG (2014) PPM1A is a RelA phosphatase with tumor suppressor-like activity. Oncogene 33(22):2918–2927. doi:10.1038/onc.2013.246
Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z (2015) A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci USA 112(16):5017–5022. doi:10.1073/pnas.1505244112
Gray PJ Jr, Prince T, Cheng J, Stevenson MA, Calderwood SK (2008) Targeting the oncogene and kinome chaperone CDC37. Nat Rev Cancer 8(7):491–495. doi:10.1038/nrc2420
Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M, Kim SJ, Ro JY, Park KM, Lee HW, Park EJ, Chun KH, Song J (2012) Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nat Commun 3:978. doi:10.1038/ncomms1981
Moquin DM, McQuade T, Chan FK (2013) CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One 8(10):e76841. doi:10.1371/journal.pone.0076841
Alameda JP, Moreno-Maldonado R, Navarro M, Bravo A, Ramirez A, Page A, Jorcano JL, Fernandez-Acenero MJ, Casanova ML (2010) An inactivating CYLD mutation promotes skin tumor progression by conferring enhanced proliferative, survival and angiogenic properties to epidermal cancer cells. Oncogene 29(50):6522–6532. doi:10.1038/onc.2010.378
Schworer SA, Smirnova II, Kurbatova I, Bagina U, Churova M, Fowler T, Roy AL, Degterev A, Poltorak A (2014) Toll-like receptor-mediated down-regulation of the deubiquitinase cylindromatosis (CYLD) protects macrophages from necroptosis in wild-derived mice. J Biol Chem 289(20):14422–14433. doi:10.1074/jbc.M114.547547
Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, Yang H, Samadi AK, Russo GL, Spagnuolo C, Ray SK, Chakrabarti M, Morre JD, Coley HM, Honoki K, Fujii H, Georgakilas AG, Amedei A, Niccolai E, Amin A, Ashraf SS, Helferich WG, Yang X, Boosani CS, Guha G, Bhakta D, Ciriolo MR, Aquilano K, Chen S, Mohammed SI, Keith WN, Bilsland A, Halicka D, Nowsheen S, Azmi AS (2015) Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. doi:10.1016/j.semcancer.2015.03.001
Acknowledgments
This work was supported by grants from the Deutsche Krebshilfe (110055) and the Deutsche Forschungsgemeinschaft (SFB 877, project B2 and Cluster of Excellence “Inflammation at Interfaces”, EXC306-PMTP1 and EXC306-PWTP2) to D. A. and a junior grant from the Medical Faculty of the Christian-Albrechts-University to S. P.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Philipp and J. Sosna contributed equally to this work.
Rights and permissions
About this article
Cite this article
Philipp, S., Sosna, J. & Adam, D. Cancer and necroptosis: friend or foe?. Cell. Mol. Life Sci. 73, 2183–2193 (2016). https://doi.org/10.1007/s00018-016-2193-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2193-2