Abstract
An increase in autoimmune diseases poses a socioeconomic challenge worldwide. Predisposing genetic risk has been identified, yet environmental factors make up a significant part of the risk in disease initiation and propagation. Next to improved hygiene and a gross reduction of infections, changes in dietary habits are one of the most evident Western lifestyle factors potentially associated with the increase in autoimmune diseases. Growing evidence suggests that particularly a typical ‘Western diet’, rich in saturated fat and salt and related pathologies can have a profound impact on local and systemic immune responses under physiologic and autoimmune conditions such as in multiple sclerosis (MS). In this review, we discuss recent findings on environmental factors influencing autoimmunity with an emphasis on the impact of ‘Western diet’ on immune homeostasis and gut microbiota in MS.
Similar content being viewed by others
Introduction
The common cause for autoimmune diseases, such as multiple sclerosis (MS), type-1 diabetes (T1D), rheumatoid arthritis (RA) or inflammatory bowel disease (IBD), is suspected to be the loss of tolerance to self. Initiating events are mostly unknown but may be associated with a broad range of overstimulated and mislead immune responses, including loss of costimulatory control by antigen presenting cells (APC) and “molecular mimicry”, which causes immune cells recognizing a certain extrinsic antigen to react also to structurally similar self-antigens [1]. Once an immune reaction is initiated, massive invasion of immune cells accompanied by a systemic/humoral immune response leads to tissue damage resulting in exposure of additional self-antigens [1, 2]. Although predisposing genetic risk factors have been identified for various autoimmune diseases, it is understood that they account only for a fraction of the overall disease [3, 4]. Hence, the remaining risk component of autoimmune diseases such as MS must be related to exogenous factors. Research efforts of the past decades confirm this finding, and it is now well accepted that the etiology of many autoimmune diseases involves environmental factors that act on top of genetic susceptibility profiles [3, 5, 6]. The increasing incidence of autoimmune diseases with a high prevalence in Western countries [7, 8] and the rapid evolution of MS in former low prevalence countries like Japan [9] nurture multiple explanatory concepts around environmental triggers. The so-called hygiene hypothesis aims to explain the increase in autoimmunity in industrialized countries by linking the decrease of infection rates and the increase in autoimmune diseases to a general improvement of hygiene standards [10].
Besides infection, there are many more environmental factors that have been proposed to promote autoimmune diseases, like MS, including climate, stress, occupation, cigarette smoking, and diet [11]. Of note, the consumption of ‘Westernized food’, including high salt, high fat, high protein, and high sugar intake, has already been associated with increasing prevalence in various diseases [12, 13]. The change of dietary habits has been under intensive investigation, revealing a direct influence on immune homeostasis and on bacterial communities colonizing the gastrointestinal tract (GIT) [14] and the gut microbiota is tightly connected to the immune system and highly involved in immune regulatory processes [15]. IBD has been associated with shifts and variety reduction in the microbiome. This observation has also been made in other autoimmune diseases not directly associated with the GIT [12, 16]. However, there is still little understanding on the mechanisms linking environmental factors to disease mechanisms, genetic predisposition, and the immune system. Gaining further insight into the influence of environment and microbiota on immune homeostasis will be a powerful source for a better understanding of the rising incidence of autoimmune pathologies with the aim to provide novel approaches for therapeutic treatment and prevention strategies.
This review will, therefore, discuss recent developments in research linking the environment to autoimmune diseases with an emphasis on the nexus of immune cells, dietary components, and gut microbiota. Thereby, we highlight the role of CD4+ T lymphocytes in MS, especially with respect to the importance of balancing effector and regulatory T cells for maintenance of immune homeostasis.
Immune cells in multiple sclerosis
Worldwide, there are an estimated 2.5 million patients suffering from MS with women twice as frequently affected as men [17]. MS is a progressive demyelinating disease characterized by disseminated central nervous system (CNS) lesions, most likely caused by an autoimmune response to CNS self-antigens [18]. Pathologically, perivascular inflammatory infiltrates in brain, optic nerve, and spinal cord dominate during the early phases of the disease. These infiltrates contain mononuclear immune cells, such as lymphocytes (CD4+ and CD8+ T cells as well as B cells), monocytes, and macrophages, and form so-called plaques, the end stage of inflammation, characterized by demyelination, astrogliosis, and neuronal as well as axonal degeneration [5, 19, 20]. Dendritic cells (DCs) have been shown to play a critical role in immune invasion of the CNS by presenting antigen to activated autoreactive T cells [21, 22]. In addition, the activation of microglia and macrophages plays an essential role in the pathogenesis of the disease [23]. Recent studies, demonstrating the presence of inflammatory cells and their products in CNS lesions, led to the generally accepted hypothesis that at least relapsing-remitting MS is triggered by pathogenic CD4+ T cells reactive against myelin constituents [5, 19, 20]. Besides these, several other cells of the innate and adaptive immune system are involved in the pathogenesis of MS. For instance, CD8+ T cells were shown to directly damage axons by the secretion of granzyme B and perforin, [24] and macrophages can contribute to tissue damage by releasing toxic molecules like nitric oxide, oxygen radicals and proinflammatory cytokines [25]. Also B cells [26–28], as well as innate lymphoid cells (ILCs) [29], γ/δ T cells [30] and NK cells [31] play distinct important roles in the autoimmune response. In particular, the recently discovered ILCs, which are tissue-resident lymphoid cells that lack specific antigen receptors, gained interest as new targets for modulating immune tolerance in autoimmune diseases like MS [29]. ILCs have been recognized for their importance in mediating the interplay between microbiota and the immune system [32], and the non-cytotoxic ILC1, ILC2, and ILC3 show a striking resemblance to CD4+ T cell subsets with respect to development and function [33, 34].
Many data on the immunopathology of MS stem from experimental autoimmune encephalomyelitis (EAE), an animal model mimicking several aspects of the disease [35–37]. EAE is most commonly induced in rodents by the immunization with myelin peptides (e.g., MOG, myelin oligodendrocyte glycoprotein) and adjuvant [38] or the transfer of myelin-reactive T cells [39]. The resulting T cell-mediated acute autoimmune reaction against myelin in the CNS induces similar symptoms to those seen in MS. It was thus initially shown in EAE models that CD4+ T helper (Th) cells play a key role in MS. MS was first thought to be a Th1 cell-mediated autoimmune disease, with interferon gamma (IFN-γ) assuming a pathogenic role, while Th2 cells producing primarily interleukin (IL)-4 or IL-10 exert a modulatory function with a protective role [40]. After the identification of the relevance of IL-23 in EAE, subsequent work showed that also Th17 cells are involved in the pathogenesis of the disease, since IL-23 is a critical growth factor for this cell subset [41, 42].
Th17 cells frequently occur in the intestine, playing a particularly important role in the intestinal immune homeostasis by providing defense against extracellular bacteria and clearance of pathogens [43]. Depending on their physiological role, Th17 cells are exposed to environmental factors and could, therefore, be influenced by nutritional components. Together with Th1 cells, Th17 cells are believed to be the main constituents of the CD4+ T effector subset that drives disease pathology in T cell-dependent autoimmune diseases [44]. However, due to the fact that Th17 cells are associated with several autoimmune diseases, recent investigations often focus on this Th cell subset [44]. In EAE studies, IL-17 deficient mice showed delayed and reduced symptoms, but no complete protection [45, 46]. More recent studies found that the pathogenicity of Th17 cells additionally depends on the IL-23-induced production of the cytokine granulocyte macrophage colony-stimulating factor (GM-CSF), probably explaining the incomplete protection from EAE in IL-17-deficient mice [47, 48]. The importance of Th17 cells was also linked to MS, as lesions of MS patients contained an increased frequency of IL-17-producing CD4+ T cells [49]. Moreover, it was found that Th17 cells are able to cross the inflamed blood–brain barrier and secrete the proinflammatory cytokine IL-17A [50]. A recent study showed that in particular Th17 instead of Th1 responses were absent in patients with MS disease abrogation after hematopoietic stem cell transplantation [51], confirming the pivotal role of Th17 cells in MS.
In contrast to effector T cells, regulatory T cells (Tregs) play a central role in immunoregulatory reactions and suppression of autoreactive immune cells. Once activated, forkhead box P3 (FoxP3) positive Tregs exert their suppressive functions via the release of anti-inflammatory cytokines like IL-10 and transforming growth factor (TGF)-β in addition to cell–cell contact-dependent mechanisms [52]. In EAE, adoptive transfer of Tregs improved disease symptoms, while ablation led to worsening of disease [52]. Importantly, an impairment of regulatory T cell function is frequently observed in patients with autoimmune diseases like MS, and it is believed to be a major cause for disruption of immune homeostasis, further contributing to autoimmune reactivity [2, 52]. The loss of Treg suppressive capacity might be related to the potential of Tregs to convert into Th1-like Tregs, secreting IFN-γ [53–55], as well as Th17-like Tregs, secreting IL-17 with a proinflammatory potential [56–59]. In MS patients, IFN-γ-secreting Tregs were found to be increased [60] and Tregs displayed lower expression levels of FoxP3 and an impaired suppressive capacity [2, 52, 61–63]. It is thus well accepted that the balance between T effector cells and Treg subsets plays a major role in autoimmune diseases like MS.
Environment and its link to MS
Genetic variants influencing susceptibility or protection from autoimmune diseases like MS have been explored in studies of twin concordance [64], familiar clustering and genome wide associations studies (GWAS) [65]. Genes account for roughly 25–30 % inheritability in monozygotic twins, and as in the majority of autoimmune diseases, variants of the human leukocyte antigen (HLA) complex provide a strong susceptibility for MS. Especially, the HLA-DRB1*1501 allele is associated with a highly elevated risk for MS development, displaying a sixfold risk increase in homozygous carriers [65]. In addition, a number of immune-related risk-alleles have been established independently of the HLA-locus, affecting in particular CD4+ T cell subset responses [66, 67]. Among the identified genes are, for instance, the IL-2 and IL-7 receptors, both genes playing a critical role for Tregs and Th effector cells [66, 67]. Moreover, predisposing variants for MS have been found for the Th17/IL-23 axis, supporting a role for Th17 cells in disease development [66]. Similar studies have also suggested an increased susceptibility caused by variants of CD86, an important coreceptor for T cell stimulation expressed by DCs, supporting the important role of DC/T cell interaction for MS [65]. A genetic link to B cell function and MS is for instance based on risk variants in the CD40 and CXCR5 genes, encoding for a surface protein inducing B cell activation and differentiation and a chemokine receptor expressed on B and T cells [28]. GWAS studies have even identified risk association with genes that are important for current and new MS therapies including vascular cell adhesion molecule 1 (VCAM1), as well as genes related to the crucial environmental factor vitamin D [65]. Nevertheless, the large amount of data on genetic predisposition for autoimmune diseases can, in most of the cases, only explain a part of the disease risk, supporting the view that the increasing prevalence of MS is triggered in addition by environmental factors. Epidemiological studies have shown a striking trend of MS toward higher prevalence with increasing latitude and an increase in disease incidence in developed countries [7, 8]. Several theories have aimed to explain these observations. Since exposure to sunlight is the main source for vitamin D synthesis in humans, a lower sunlight exposure in high-risk regions such as Northern Europe results in a decreased generation of vitamin D. In fact, patients with MS were found to have lower blood levels of vitamin D [68, 69] and also in other autoimmune diseases, low vitamin D levels may represent an emerging risk factor [70, 71]. Studies on the effect of vitamin D in autoimmunity suggest an immunomodulatory capacity with anti-inflammatory action [72, 73]. Moreover, UVB light increased levels of tolerogenic DCs and Tregs while reducing effector T cell counts in MS patients [74]. In addition to sunlight, fatty fish could be a dietary source of vitamin D, and fish consumption correlated with lower MS prevalence in coastal areas [75]. However, the recent OFAMS study failed to detect any influence of fish oil on the course of MS [76]. Nevertheless, there is an increasing amount of data demonstrating the positive role of vitamin D or other vitamins like biotin [77] in MS, as summarized elsewhere in depth [78–80].
Furthermore, the hygiene hypothesis states that individuals not exposed to certain infections early in life but growing up with improved hygiene may develop a hyperalert immune system, favoring the occurrence of autoimmune diseases [10, 81]. While according to the hygiene hypothesis some infectious agents may be protective, others may increase the risk, such as Epstein–Barr virus (EBV) which is associated with MS especially when infection takes place in late adolescence [5]. A potential explanation for that might be the similarity of the EBV nuclear antigen (EBNA)-1 to myelin surface proteins, leading to crossreactivity of adaptive immune cells [82]. Interestingly, T cells specific to EBNA-1 display a significantly higher frequency as well as a broader specificity in MS patients than in healthy controls [83]. Although associations between MS and EBV infection have been well investigated, the role of EBV in MS pathology remains unclear as further studies could not demonstrate latent or active EBV infection in active MS lesions [84]. Infections that might confer a protective effect to the host are colonizations of the GIT by parasitic worms. Different studies have shown that helminths may affect the host’s immune response by the promotion of an anti-inflammatory environment [85, 86]. Based on this, the possibility of treating MS with helminths has already been explored in animal models [87–89] and also phase 1 clinical trials [90]. In line with these findings, the fact that colonization of the GIT by parasitic worms has decreased over the past decades in industrialized countries [91] possibly contributes to an increasing MS prevalence.
The gut and its microbiota recently gained a lot of attention in various fields of research. The human gut microbiome is expected to consist of more than 1014 bacterial cells from about 500–1000 species and helps the host to maintain the body in homeostasis [92]. The first exposure to the human microbiome occurs during birth, and breast milk or formula feeding further influences the colonization of the new born’s own gut microbiome [93–95]. During a lifetime, the human gut is recolonized permanently over the years with dominating bacterial orders, such as Firmicutes and Bacteroidetes, and other bacteria found in minor amounts, creating a unique human gut microbiota for each individual [16]. The human microbiota reaches maximum diversity at adolescence and can then be stable for years. A large amount of physiological functions, such as food digestion (providing fermentation products) and competition with potential pathogens, have been described for the commensal gut bacteria [96, 97]. Besides this, a vast collection of data points out that the gut microbiota is essential for the proper function of our immune system and metabolism and thus has a strong impact on human’s health. Indeed, changes in the gut microbiota have been observed in several diseases such as IBD [98], allergies [99], and asthma [100–102]. Those changes are induced by many factors, such as diet, stress or medication, and can lead to a so-called dysbiosis [103, 104]. A common consequence of dysbiosis is the alteration of the mucosal immune system leading to a rise of gut inflammation and alterations of intestinal immunity [105]. It was shown that a dysbiotic microbiome could lead to Treg deficiency and an activation of proinflammatory Th17 cells [106, 107]. While not expecting a direct correlation between the gut and brain autoimmunity, EAE studies indicate that the microbiota might play a role in MS as well. For instance, germ-free mice were shown to be protected from EAE induction, and using transgenic mice, Berer and colleagues could show that gut bacteria are a necessary prerequisite to induce a relapsing-remitting autoimmune disease [108]. Consistent with this, oral antibiotic treatment reduced and modulated bacterial populations in wild-type mice and, thereby, significantly ameliorated EAE onset and severity [109]. This effect was correlated with an increase in IL-10 producing Tregs, mainly induced by polysaccharide A of Bacteroides fragilis [110, 111]. Similar to Tregs, Th17 cells could be influenced by the gut microbiota. It was demonstrated that distinct species of commensal bacteria, namely, segmented filamentous bacteria (SFB), can specifically induce Th17 cells [112]. It was further shown that the induction of these cells could be indirectly influenced by luminal adenosine triphosphate (ATP) secreted from bacteria [113]. Moreover, the recently described gut–brain-axis may further support a possible link between gut microbiota and autoimmunity [114].
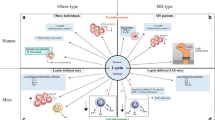
In addition to direct effects of the microbiota, also dietary changes in association with a microbiota modulation are likely to be linked with the increasing incidences of autoimmune disorders [115]. It was shown that a ‘Western diet’ increases inflammation and might negatively affect the gut-immune homeostasis. In contrast, low-calorie diets based on fruits, fish and vegetables downregulate proinflammatory molecules and restore or maintain a healthy symbiotic gut microbiota [116]. For instance, plant-derived nutrients were found to be associated with an anti-inflammatory potential by acting as ligands of the aryl hydrocarbon receptor (AhR) [117, 118]. AhR acts as a transcription factor in a variety of immune cells, including Th17 and Tregs, and has been associated with susceptibility as well as prevention of autoimmune diseases depending on its ligands [107, 117, 118]. In that matter, indole-3-carbinol, deriving from crucifers such as broccoli, has been shown to suppress the production of proinflammatory cytokines [118, 119], whereas the tryptophan-derived AhR ligand FICZ (6-Formylindolo(3,2-b)carbazole) specifically increases the Th17 population and, therefore, worsens EAE severity [107]. Another plant-derived component supporting the induction of Tregs is retinoic acid (RA). RA is metabolized from vitamin A, which is derived from carotenoids in plants and as retinol from animals [118]. The highly metabolically active intermediate product all-trans RA (ATRA) can be generated by mucosal DCs potent in inducing and maintaining regulatory T cells [120, 121]. Nutrients, mostly discussed as risk factors in autoimmunity, are related to a ‘Western diet’. In that matter, especially, changes in the gut microbiome in association with diets rich in fat and salt have gained increasing attention during recent years and are discussed as potential risk factors for MS (Fig. 1).
Fatty acids
Integral components of the daily diet that have recently been linked to autoimmunity via changes in the gut microbiota are fatty acids. Excessive fat intake, in general, is a prominent factor inducing obesity. Associations between obesity and MS have already been demonstrated, displaying a positive correlation between body mass index and the risk of developing MS, especially at younger ages [122]. Obesity, defined by the inadequate accumulation of white adipose tissue (WAT), can lead to a state of systemic inflammation called “metaflammation”. Metaflammation occurs, because WAT is not only involved in energy storage, but also functions as an endocrine organ secreting proinflammatory tumor necrosis factor (TNF)-α, IL-6 or leptin. The latter in particular is a cytokine-like hormone profoundly influencing T cell responses in EAE [123–125]. Leptin was shown to enhance phagocytosis and cytokine secretion in macrophages and to promote CD4+ T cell proliferation and survival [126], favoring Th1 and Th17 reactions while inhibiting Treg responses [125–127]. In MS patients, monocytes and T cells present in MS lesions and patient-derived cerebrospinal fluid (CSF) both highly express leptin and leptin receptor [128, 129]. However, MS incidence is not necessarily accompanied by weight gain, thus suspecting a direct effect of fatty acids on immunity. Fatty acids, subdivided into saturated and unsaturated fatty acids, were first correlated with MS in the 1950s [130, 131]. More recent work related ω-3 polyunsaturated fatty acids (PUFAs) to anti-inflammatory effects [132]. In contrast, ω-6 PUFAs are precursors of proinflammatory eicosanoids that may promote the activation of the Th17 pathway [133] and are thus suspected to play a detrimental role in a variety of diseases. Studies in the EAE model demonstrated saturated fats to be a risk or beneficial factor depending on their chain length [134]. The increased intake of medium- and long-chain fatty acids (MCFA and LCFA) by the consumption of an experimental ‘Western diet’ was shown to exacerbate autoimmunity in the CNS [134]. This was due to an increased infiltration of Th1 and Th17 cells in the spinal cord. In vitro, the differentiation of murine and human CD4+ T cells into Th1 and Th17 cells was significantly increased by the addition of LCFAs. In parallel, the generation of Tregs was suppressed, coinciding with a decreased secretion of anti-inflammatory cytokines. In murine EAE, diets rich in LCFA were shown to modulate the microbiome, such that naïve CD4+ T cells are exposed to increased LCFA in the small intestine, thus inducing more proinflammatory T cell responses [134].
Short-chain fatty acids (SCFA) with chain lengths reaching from one to five C atoms mostly occur in the gut as fermentation products of dietary fibers by commensal bacteria [135]. A decrease in SCFA has been observed in patients with IBD [99, 136]. In MS patients, levels of Clostridia clusters XIVa and IV were shown to be reduced [137], both formed by diverse bacterial species that are able to produce SCFA such as butyrate [138, 139]. Butyrate displays anti-inflammatory properties, probably indicating that a reduction of these microbes in MS patients may be associated with disease [137–140]. Most data discussing the mechanism for the effect of SCFA demonstrate the involvement of Tregs. In a murine model of IBD, the administration of acetate (C2:0), propionate (C3:0) or butyrate (C4:0) increased the level of Tregs in the gut [136, 141]. In addition, the administration of butyrate to germ-free mice mimicked the effect of Clostridium colonization and increased Treg levels in colon lamina propria [142]. Investigating the effects of SCFA in the EAE model also revealed an increase of Tregs, while suppressing the differentiation of Th17 cells [134]. In EAE, feeding propionate ameliorated the disease by promoting Tregs in the small intestine. As a possible mechanism for how propionate might regulate the differentiation of Tregs is the acetylation of histone H3. SCFA, most potently butyrate, were shown to function as histone deacetylase inhibitors, maintaining acetylation of genes important for Treg function, such as Foxp3 [141–143]. Whether this in vitro effect could also explain the in vivo amelioration of EAE remains unclear. However, synthetic small inhibitors of histone deacetylases have already been shown to decrease inflammation in animal models of arthritis, IBD, asthma, diabetes, cardiovascular diseases, and MS [143]. Thus, SCFA as naturally occurring nutrients [144] or fermentation products may have a possible therapeutic value for autoimmune diseases like MS by potentially triggering the production of anti-inflammatory Tregs.
Salt
Another typical hallmark of ‘Westernized food’ is the high content of sodium chloride (NaCl). In particular, processed or so-called ‘fast foods’ may contain significantly more NaCl than homemade meals [145]. Recent literature links this high sodium intake to cardiovascular diseases [146], cancer [147], chronic inflammation [148], and also autoimmune diseases [6, 149, 150]. It was demonstrated that primary and secondary lymphoid organs, such as the thymus and lymph nodes, show increased hypertonicity in comparison to the blood [151], indicating that immune cells have to cope with these changes when infiltrating peripheral tissues or during activation in secondary lymphoid organs. Studies dating back in the early 1990s have demonstrated that the activation of innate and adaptive immune cells under hypertonic conditions could enhance immune function, and monocytes were shown to get highly proinflammatory [152], while T cell lines were shown to secrete less anti-inflammatory factors and to produce, for instance, more TNF-α [153]. Moreover, it was recently shown that high salt conditions, mimicking in vivo situations in the tissue after a high salt diet, enhanced the activation of classical M1 macrophages, and increased the expression of proinflammatory mediators [154–156]. In contrast to this, excess salt diminished the activation of the so-called M2 macrophages (M(IL-4 + IL-13)) and decreased their ability to suppress effector T cell proliferation [157]. Similar to cells of the innate immune system, raising the sodium concentrations in vitro also affected adaptive immune cells and promoted the differentiation of murine and human Th17 cells with a pathogenic phenotype, displaying increased expression of, e.g., CSF2 and IL-23R [158, 159]. It was demonstrated that this effect was linked to p38/mitogen-activated protein kinase (MAPK), nuclear factor of activated T cells 5 (NFAT5), and serum- and glucocorticoid-regulated kinase-1 (SGK1) dependent pathways. The fact that in particular pathogenic Th17 cells with a similar phenotype are involved in autoimmune diseases indicates that a high salt diet may represent a previously unrecognized environmental risk factor for autoimmunity. Indeed, in the EAE model, a high salt diet augmented disease onset and severity [158, 159]. Exacerbated disease was accompanied by increased induction of Th17 cells and heightened numbers of CNS infiltrating pathogenic Th17 cells. More recent work focusing on the effects of sodium chloride on regulatory immune cells provided evidence that high salt conditions almost completely block the suppressive function of human and murine Tregs in vitro and in vivo. High amounts of salt induced Th1-like Tregs, secreting IFN-γ in an SGK1-dependent manner [160]. In MS patients, a recently published observational study demonstrated a heightened disease activity and enhanced inflammation in subjects with an increased dietary sodium intake [149]. However, direct human data on effects of high salt intake on the immune system are still sparse. A few available studies indicate a heightened immune activation in association with excess salt intake. In particular, the composition of monocyte subsets was shown to be shifted towards higher numbers of proinflammatory monocytes by a short-term increase in dietary salt intake [161]. Similarly, a recent longitudinal study observed a striking correlation of monocyte numbers and function with salt-intake levels. The consumption of a high salt diet was paralleled by higher monocyte counts and a significant increase in IL-6 and IL-23 production, whereas the secretion of IL-10 was decreased [162]. Intriguingly, IL-6 and IL-23 are major inducers of Th17 cells also in humans. Novel strategies to investigate the body’s sodium content recently showed that a high salt diet is accompanied by a periodical storage of sodium in skin interstitium and the muscle [163–165]. This process represents a new regulatory mechanism independent from water retention and sodium clearance by the kidney. Instead, this extra-renal sodium clearance was shown to involve the immune system. Skin-resident macrophages, activated in an NFAT5-dependent manner, secrete vascular endothelial growth factor c (VEGF-C), a growth factor for lymphatic vessels and, thereby, inhibit the development of salt-mediated hypertension [164]. Initial studies, measuring the sodium content in humans by 23Na-MRI, revealed this specific accumulation of sodium in the skin and muscle [163, 166]. The concept that higher interstitial sodium content via increases in dietary salt intake may drive proinflammatory responses of the innate immune system, promoting Th17 cell induction was recently supported by a study of Medzhitov and colleagues. In this study, it was shown that macrophages could sense hypertonic conditions through the caspase-1 pathway, thereby promoting Th17 cell induction by heightened IL-1β secretion [167]. In line with these findings, Jantsch et al. demonstrated that sodium, which accumulated at the site of skin infections, was able to boost proinflammatory macrophage responses in a p38/MAPK and NFAT5-dependent manner [156]. In summary, these data indicate that the sodium balance may affect innate and adaptive immune function and homeostasis at various levels. Salt intake, therefore, might represent an environmental risk factor, which may influence autoimmunity by promoting proinflammatory and blocking anti-inflammatory immune responses. However, it remains to be seen how significant these effects are for human autoimmunity. It is also likely that the genetic architect could play a role here, as recently indicated by an EAE study [168]. Moreover, alterations in the gut microbiota may indirectly contribute to the observed effects of altered immune function and disease [2, 6].
Conclusion
Increasing research efforts indicate that nutritional factors have the capability to potently modulate autoimmune responses and inflammation. Sodium chloride and saturated fatty acids have been implicated as risk factors in many diseases, such as stroke, hypertension, cardiovascular diseases, chronic inflammation and autoimmunity. It is thus not surprising that there is growing interest in special diets as add-on therapies to conventional therapeutics. Although no long-term clinical trials currently exist, the data discussed above suggest that the impact of components of the daily diet like saturated fats and sodium on inflammatory processes and autoimmunity should be further investigated.
The gut as the main absorption interface for nutritional components displays the most prominent anatomic site that links diet and disease. Recent studies have already suggested a role for the gut microbiome in a number of diseases, including T1D, IBD, and obesity, and present further evidence that the gut microbiome may also play a role in diseases affecting the CNS, such as MS. The impact of gut microbiota and its metabolites on the mucosal immune system was shown to not only affect the gut environment, but also to modulate extra-intestinal immune responses by influencing the balance of pro- and anti-inflammatory T cell subsets. Modifying the gut microbiota, either directly or indirectly through dietary factors, might thus be a potential therapeutic option for the treatment of various diseases, including MS. Promoting the induction of anti-inflammatory Tregs and reducing pathogenic Th17 cell responses might represent here the most promising strategy in the context of autoimmunity.
References
Rosenblum MD, Remedios KA, Abbas AK (2015) Mechanisms of human autoimmunity. J Clin Invest 125(6):2228–2233
Kleinewietfeld M, Hafler DA (2013) The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol 25(4):305–312
Bogdanos DP, Smyk DS, Rigopoulou EI, Mytilinaiou MG, Heneghan MA, Selmi C et al (2012) Twin studies in autoimmune disease: genetics, gender and environment. J Autoimmun 38(2–3):J156–J169
Marson A, Housley WJ, Hafler DA (2015) Genetic basis of autoimmunity. J Clin Invest 125(6):2234–2241
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517
Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M (2014) Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep 14(1):404
Organization WH, Federation MSI (2008) Atlas: multiple sclerosis resources in the world 2008. Cited 2016 Jan 20. http://www.who.int/iris/handle/10665/43968
Bach J-F (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347(12):911–920
Houzen H, Niino M, Hata D, Nakano F, Kikuchi S, Fukazawa T et al (2008) Increasing prevalence and incidence of multiple sclerosis in northern Japan. Mult Scler Houndmills Basingstoke Engl 14(7):887–892
Fleming J, Fabry Z (2007) The hygiene hypothesis and multiple sclerosis. Ann Neurol 61(2):85–89
Marrie RA (2004) Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol 3(12):709–718
Thorburn AN, Macia L, Mackay CR (2014) Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40(6):833–842
Odegaard AO, Koh WP, Yuan J-M, Gross MD, Pereira MA (2012) Western-style fast food intake and cardiometabolic risk in an Eastern country. Circulation 126(2):182–188
Conlon MA, Bird AR (2015) The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7(1):17–44
Kuhn KA, Stappenbeck TS (2013) Peripheral education of the immune system by the colonic microbiota. Semin Immunol 25(5):364–369
Salonen A, de Vos WM (2014) Impact of diet on human intestinal microbiota and health. Annu Rev Food Sci Technol 5:239–262
Atlas-of-MS.pdf [Internet]. Cited 2016 Feb 9. http://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf
Haghikia A, Hohlfeld R, Gold R, Fugger L (2013) Therapies for multiple sclerosis: translational achievements and outstanding needs. Trends Mol Med 19(5):309–319
Hohlfeld R, Dornmair K, Meinl E, Wekerle H (2015) The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol
Hohlfeld R, Dornmair K, Meinl E, Wekerle H (2015) The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol
Lande R, Gafa V, Serafini B, Giacomini E, Visconti A, Remoli ME et al (2008) Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol 67(5):388–401
Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi GL et al (2006) Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol 65(2):124–141
Jack C, Ruffini F, Bar-Or A, Antel JP (2005) Microglia and multiple sclerosis. J Neurosci Res 81(3):363–373
Irani DN (2005) Immunological mechanisms in multiple sclerosis. Clin Appl Immunol Rev 5(4):257–269
Zindler E, Zipp F (2010) Neuronal injury in chronic CNS inflammation. Best Pract Res Clin Anaesthesiol 24(4):551–562
Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M et al (2013) MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med 210(13):2921–2937
Krumbholz M, Derfuss T, Hohlfeld R, Meinl E (2012) B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol 8(11):613–623
Disanto G, Morahan JM, Barnett MH, Giovannoni G, Ramagopalan SV (2012) The evidence for a role of B cells in multiple sclerosis. Neurology 78(11):823–832
Degn M, Modvig S, Dyring-Andersen B, Bonefeld CM, Frederiksen JL, Geisler C et al (2015) Increased prevalence of lymphoid tissue inducer cells in the cerebrospinal fluid of patients with early multiple sclerosis. Mult Scler Houndmills Basingstoke Engl
Schirmer L, Rothhammer V, Hemmer B, Korn T (2013) Enriched CD161high CCR6 + γδ T cells in the cerebrospinal fluid of patients with multiple sclerosis. JAMA Neurol 70(3):345–351
Rodríguez-Martín E, Picón C, Costa-Frossard L, Alenda R, Sainz de la Maza S, Roldán E et al (2015) Natural killer cell subsets in cerebrospinal fluid of patients with multiple sclerosis. Clin Exp Immunol 180(2):243–249
Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y et al (2014) Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343(6178):1249288
Artis D, Spits H (2015) The biology of innate lymphoid cells. Nature 517(7534):293–301
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G et al (2013) Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 13(2):145–149
Gold R, Linington C, Lassmann H (2006) Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain J Neurol 129(Pt 8):1953–1971
Steinman L, Zamvil SS (2005) Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol 26(11):565–571
Steinman L, Zamvil SS (2006) How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol 60(1):12–21
Stromnes IM, Goverman JM (2006) Active induction of experimental allergic encephalomyelitis. Nat Protoc 1(4):1810–1819
Stromnes IM, Goverman JM (2006) Passive induction of experimental allergic encephalomyelitis. Nat Protoc 1(4):1952–1960
Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T et al (2002) Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415(6871):536–541
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B et al (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421(6924):744–748
Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA et al (2003) Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 198(12):1951–1957
Huber S, Gagliani N, Flavell RA (2012) Life, death, and miracles: Th17 cells in the intestine. Eur J Immunol 42(9):2238–2245
Korn T, Bettelli E, Oukka M, Kuchroo VK (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27:485–517
Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S et al (2006) IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol Baltim Md 1950 177(1):566–73
Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B et al (2009) IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest 119(1):61–69
El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F et al (2011) The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 12(6):568–575
Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L et al (2011) RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12(6):560–567
Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM et al (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172(1):146–155
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M et al (2007) Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13(10):1173–1175
Darlington PJ, Touil T, Doucet J-S, Gaucher D, Zeidan J, Gauchat D et al (2013) Diminished Th17 (not Th1) responses underlie multiple sclerosis disease abrogation after hematopoietic stem cell transplantation. Ann Neurol 73(3):341–354
Kleinewietfeld M, Hafler DA (2014) Regulatory T cells in autoimmune neuroinflammation. Immunol Rev 259(1):231–244
Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M et al (2009) Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 10(9):1000–1007
Duarte JH, Zelenay S, Bergman M-L, Martins AC, Demengeot J (2009) Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol 39(4):948–955
Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT (2004) Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol 5(11):1149–1156
Xu L, Kitani A, Fuss I, Strober W (2007) Cutting edge: regulatory T cells induce CD4+ CD25-Foxp3-T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol Baltim Md 1950 178(11):6725–9
Koenen HJPM, Smeets RL, Vink PM, van Rijssen E, Boots AMH, Joosten I (2008) Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112(6):2340–2352
Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G et al (2008) DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol 38(12):3274–3281
Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP et al (2008) Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29(1):44–56
Dominguez-Villar M, Baecher-Allan CM, Hafler DA (2011) Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med 17(6):673–675
Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens J-L et al (2008) Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 123(1):79–89
Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA (2004) Loss of functional suppression by CD4+ CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 199(7):971–979
Frisullo G, Nociti V, Iorio R, Patanella AK, Caggiula M, Marti A et al (2009) Regulatory T cells fail to suppress CD4T+-bet+ T cells in relapsing multiple sclerosis patients. Immunology 127(3):418–428
Hawkes CH, Macgregor AJ (2009) Twin studies and the heritability of MS: a conclusion. Mult Scler 15(6):661–667
International Multiple Sclerosis Genetics Consortium, Wellcome Trust Case Control Consortium 2, Sawcer S, Hellenthal G, Pirinen M, Spencer CCA et al (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476(7359):214–9
Nylander A, Hafler DA (2012) Multiple sclerosis. J Clin Invest 122(4):1180–1188
The International Multiple Sclerosis Genetics Consortium (2007) Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357(9):851–862
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A (2006) Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296(23):2832–2838
Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH et al (2014) Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol 71(3):306–314
Holick MF (2005) Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J 98(10):1024–1027
Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG et al (2004) Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa women’s health study. Arthritis Rheum 50(1):72–77
Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R (2006) Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83(4):754–759
Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L (2001) Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol Baltim Md 1950 167(4):1945–53
Breuer J, Schwab N, Schneider-Hohendorf T, Marziniak M, Mohan H, Bhatia U et al (2014) Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann Neurol 75(5):739–758
Bäärnhielm M, Olsson T, Alfredsson L (2014) Fatty fish intake is associated with decreased occurrence of multiple sclerosis. Mult Scler Houndmills Basingstoke Engl 20(6):726–732
Torkildsen O, Wergeland S, Bakke S, Beiske AG, Bjerve KS, Hovdal H et al (2012) ω-3 fatty acid treatment in multiple sclerosis (OFAMS Study): a randomized, double-blind, placebo-controlled trial. Arch Neurol 69(8):1044–1051
Sedel F, Papeix C, Bellanger A, Touitou V, Lebrun-Frenay C, Galanaud D et al (2015) High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord 4(2):159–169
VanAmerongen BM, Dijkstra CD, Lips P, Polman CH (2004) Multiple sclerosis and vitamin D: an update. Eur J Clin Nutr 58(8):1095–1109
Simon KC, Munger KL, Ascherio A (2012) Vitamin D and multiple sclerosis: epidemiology, immunology, and genetics. Curr Opin Neurol 25(3):246–251
Ascherio A, Munger KL, Simon KC (2010) Vitamin D and multiple sclerosis. Lancet Neurol 9(6):599–612
Okada H, Kuhn C, Feillet H, Bach J-F (2010) The, “hygiene hypothesis” for autoimmune and allergic diseases: an update. Clin Exp Immunol 160(1):1–9
Ascherio A, Munger KL (2007) Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol 61(4):288–299
Lünemann JD, Edwards N, Muraro PA, Hayashi S, Cohen JI, Münz C et al (2006) Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain J Neurol 129(Pt 6):1493–1506
Sargsyan SA, Shearer AJ, Ritchie AM, Burgoon MP, Anderson S, Hemmer B et al (2010) Absence of Epstein–Barr virus in the brain and CSF of patients with multiple sclerosis. Neurology 74(14):1127–1135
Versini M, Jeandel P-Y, Bashi T, Bizzaro G, Blank M, Shoenfeld Y (2015) Unraveling the Hygiene Hypothesis of helminthes and autoimmunity: origins, pathophysiology, and clinical applications. BMC Med 13:81
Ben-Ami Shor D, Harel M, Eliakim R, Shoenfeld Y (2013) The hygiene theory harnessing helminths and their ova to treat autoimmunity. Clin Rev Allergy Immunol 45(2):211–6
La Flamme AC, Ruddenklau K, Bäckström BT (2003) Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun 71(9):4996–5004
Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L (2010) Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol 32(6):450–459
Kuijk LM, Klaver EJ, Kooij G, van der Pol SMA, Heijnen P, Bruijns SCM et al (2012) Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol 51(2):210–218
Fleming JO, Isaak A, Lee JE, Luzzio CC, Carrithers MD, Cook TD et al (2011) Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler Houndmills Basingstoke Engl 17(6):743–754
Weinstock JV, Summers R, Elliott DE (2004) Helminths and harmony. Gut 53(1):7–9
Neish AS (2009) Microbes in gastrointestinal health and disease. Gastroenterology 136(1):65–80
Fanaro S, Chierici R, Guerrini P, Vigi V (2003) Intestinal microflora in early infancy: composition and development. Acta Paediatr Oslo Nor 1992 Suppl 91(441):48–55
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I et al (2006) Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118(2):511–521
Marcobal A, Sonnenburg JL (2012) Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 18(Suppl 4):12–15
Louis P, O’Byrne CP (2010) Life in the gut: microbial responses to stress in the gastrointestinal tract. Sci Prog 93(Pt 1):7–36
Kamada N, Chen GY, Inohara N, Núñez G (2013) Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14(7):685–690
Wang W, Jovel J, Halloran B, Wine E, Patterson J, Ford G et al (2015) Metagenomic analysis of microbiome in colon tissue from subjects with inflammatory bowel diseases reveals interplay of viruses and bacteria. Inflamm Bowel Dis 21(6):1419–1427
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C et al (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20(2):159–166
Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ et al (2015) Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 6:7320
Brown EM, Sadarangani M, Finlay BB (2013) The role of the immune system in governing host-microbe interactions in the intestine. Nat Immunol 14(7):660–667
Clemente JC, Ursell LK, Parfrey LW, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270
Galley JD, Bailey MT (2014) Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes 5(3):390–396
Peterson CT, Sharma V, Elmén L, Peterson SN (2015) Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol 179(3):363–377
Chassaing B, Gewirtz AT (2014) Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol 42(1):49–53
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM et al (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57(6):1470–1481
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld J-C et al (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453(7191):106–109
Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C et al (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479(7374):538–541
Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S et al (2009) Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol Baltim Md 1950 183(10):6041–50
Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL et al (2010) A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol 3(5):487–495
Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S et al (2010) Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol Baltim Md 1950 185(7):4101–4108
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U et al (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139(3):485–498
Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M et al (2008) ATP drives lamina propria T(H)17 cell differentiation. Nature 455(7214):808–812
Mayer EA (2011) Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 12(8):453–466
Riccio P, Rossano R (2015) Nutrition facts in multiple sclerosis. ASN Neuro 7(1)
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S et al (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107(33):14691–14696
Nguyen NT, Nakahama T, Le DH, Van Son L, Chu HH, Kishimoto T (2014) Aryl hydrocarbon receptor and kynurenine: recent advances in autoimmune disease research. Front Immunol 5:551
Veldhoen M, Brucklacher-Waldert V (2012) Dietary influences on intestinal immunity. Nat Rev Immunol 12(10):696–708
Benson JM, Shepherd DM (2011) Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatory effects on murine dendritic cells. Toxicol Sci Off J Soc Toxicol 124(2):327–338
Bakdash G, Vogelpoel LTC, van Capel TMM, Kapsenberg ML, de Jong EC (2015) Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol 8(2):265–278
Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q et al (2014) Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA 111(33):E3432–E3440
Hedström AK, Olsson T, Alfredsson L (2012) High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler J 18(9):1334–1336
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11(2):85–97
Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D et al (2009) Obesity predisposes to Th17 bias. Eur J Immunol 39(9):2629–2635
Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394(6696):897–901
La Cava A, Matarese G (2004) The weight of leptin in immunity. Nat Rev Immunol 4(5):371–379
Yu Y, Liu Y, Shi F-D, Zou H, Matarese G, La Cava A (2013) Cutting edge: leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J Immunol Baltim Md 1950 190(7):3054–3058
Frisullo G, Mirabella M, Angelucci F, Caggiula M, Morosetti R, Sancricca C et al (2007) The effect of disease activity on leptin, leptin receptor and suppressor of cytokine signalling-3 expression in relapsing-remitting multiple sclerosis. J Neuroimmunol 192(1–2):174–183
Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H et al (2002) Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8(5):500–508
Swank RL, Goodwin JW (2003) How saturated fats may be a causative factor in multiple sclerosis and other diseases. Nutr Burbank Los Angel Cty Calif 19(5):478
Swank RL (1950) Multiple sclerosis; a correlation of its incidence with dietary fat. Am J Med Sci 220(4):421–430
Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C et al (2013) Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38(6):1154–1163
Sakata D, Yao C, Narumiya S (2010) Prostaglandin E2, an immunoactivator. J Pharmacol Sci 112(1):1–5
Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A et al (2015) Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43(4):817–829
Evans MA, Shronts EP (1992) Intestinal fuels: glutamine, short-chain fatty acids, and dietary fiber. J Am Diet Assoc 92(10):1239–46, 1249
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M et al (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341(6145):569–573
Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T et al (2015) Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One 10(9):e0137429
Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H et al (2013) Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500(7461):232–236
Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y et al (2011) Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331(6015):337–341
Säemann MD, Böhmig GA, Osterreicher CH, Burtscher H, Parolini O, Diakos C et al (2000) Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J Off Publ Fed Am Soc Exp Biol 14(15):2380–2382
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504(7480):446–450
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504(7480):451–455
Wang L, de Zoeten EF, Greene MI, Hancock WW (2009) Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nat Rev Drug Discov 8(12):969–981
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28(10):1221–1227
Brown IJ, Tzoulaki I, Candeias V, Elliott P (2009) Salt intakes around the world: implications for public health. Int J Epidemiol 38(3):791–813
Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE et al (2014) Global sodium consumption and death from cardiovascular causes. N Engl J Med 371(7):624–634
D’Elia L, Galletti F, Strazzullo P (2014) Dietary salt intake and risk of gastric cancer. Cancer Treat Res 159:83–95
Sundström B, Johansson I, Rantapää-Dahlqvist S (2015) Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: results from a nested case-control study. Rheumatol Oxf Engl 54(3):487–493
Farez MF, Fiol MP, Gaitán MI, Quintana FJ, Correale J (2015) Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 86(1):26–31
Hucke S, Wiendl H, Klotz L (2015) Implications of dietary salt intake for multiple sclerosis pathogenesis. Mult Scler Houndmills Basingstoke Engl
Go WY, Liu X, Roti MA, Liu F, Ho SN (2004) NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA 101(29):10673–10678
Shapiro L, Dinarello CA (1995) Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci USA 92(26):12230–12234
Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, Chen Y et al (2010) Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol 88(6):1181–1189
Müller S, Quast T, Schröder A, Hucke S, Klotz L, Jantsch J et al (2013) Salt-dependent chemotaxis of macrophages. PloS One 8(9):e73439
Zhang W-C, Zheng X-J, Du L-J, Sun J-Y, Shen Z-X, Shi C et al (2015) High salt primes a specific activation state of macrophages, M(Na). Cell Res 25(8):893–910
Jantsch J, Schatz V, Friedrich D, Schröder A, Kopp C, Siegert I et al (2015) Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 21(3):493–501
Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W et al (2015) High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest 125(11):4223–4238
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA et al (2013) Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496(7446):518–522
Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y et al (2013) Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496(7446):513–517
Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N et al (2015) Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest 125(11):4212–4222
Zhou X, Zhang L, Ji W-J, Yuan F, Guo Z-Z, Pang B et al (2013) Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte-platelet aggregates in humans: implications in end organ inflammation. PLoS One 8(4):e60332
Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I et al (2015) Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res J Lab Clin Med 166(1):103–110
Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN et al (2013) 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 61(3):635–640
Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K et al (2009) Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15(5):545–552
Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV et al (2013) Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123(7):2803–2815
Linz P, Santoro D, Renz W, Rieger J, Ruehle A, Ruff J et al (2015) Skin sodium measured with 23Na MRI at 7.0 T. NMR Biomed 28(1):54–62
Ip WKE, Medzhitov R (2015) Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun 6:6931
Krementsov DN, Case LK, Hickey WF, Teuscher C (2015) Exacerbation of autoimmune neuroinflammation by dietary sodium is genetically controlled and sex specific. FASEB J Off Publ Fed Am Soc Exp Biol 29(8):3446–3457
Acknowledgments
RAL holds an endowed professorship supported by the Novartis Foundation. MK was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (640116) and by a SALK-grant from the government of Flanders, Belgium.
Author information
Authors and Affiliations
Corresponding author
Additional information
Stefanie Jörg, Diana A. Grohme and Melanie Erzler contributed equally.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jörg, S., Grohme, D.A., Erzler, M. et al. Environmental factors in autoimmune diseases and their role in multiple sclerosis. Cell. Mol. Life Sci. 73, 4611–4622 (2016). https://doi.org/10.1007/s00018-016-2311-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2311-1