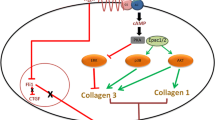
Abstract
Fibrosis is a pathological form of aberrant tissue repair, the complications of which account for nearly half of all deaths in the industrialized world. All tissues are susceptible to fibrosis under particular pathological sets of conditions. Though each type of fibrosis has characteristics and hallmarks specific to that particular condition, there appear to be common factors underlying fibrotic diseases. One of these ubiquitous factors is the paradigm of the activated myofibroblast in the promotion of fibrotic phenotypes. Recent research has implicated metabolic byproducts of the amino acid tryptophan, namely serotonin and kynurenines, in the pathology or potential pharmacologic therapy of fibrosis, in part through their effects on development of myofibroblast phenotypes. Here, we review literature underlying what is known mechanistically about the effects of these compounds and their respective pathways on fibrosis. Pharmacologic administration of kynurenine improves scarring outcomes in vivo likely not only through its well-characterized immunosuppressive properties but also via its demonstrated antagonism of fibroblast activation and of collagen deposition. In contrast, serotonin directly promotes activation of fibroblasts via activation of canonical TGF-β signaling, and overstimulation with serotonin leads to fibrotic outcomes in vivo. Recently discovered feedback inhibition between serotonin and kynurenine pathways also reveals more information about the cellular physiology of tryptophan metabolism and may also underlie possible paradigms for anti-fibrotic therapy. Together, understanding of the effects of tryptophan metabolism on modulation of fibrosis may lead to the development of new therapeutic avenues for treatment through exploitation of these effects.






Similar content being viewed by others
References
Wynn TA (2007) Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Investig 117:524–529
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Walraven M, Hinz B (2018) Therapeutic approaches to control tissue repair and fibrosis: extracellular matrix as a game changer. Matrix Biol. https://doi.org/10.1016/j.matbio.2018.02.020
Hinz B (2016) Myofibroblasts. Exp Eye Res 142:56–70
Friedman SL, Sheppard D, Duffield JS, Violette S (2013) Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 5:167sr1
Darby IA, Laverdet B, Bonté F, Desmoulière A (2014) Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 7:301
Klingberg F et al (2014) Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J Cell Biol 207:283–297
Froese AR et al (2016) Stretch-induced activation of transforming growth factor-β1 in pulmonary fibrosis. Am J Respir Crit Care Med 194:84–96
Ploeger DT, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA (2013) Cell plasticity in wound healing: paracrine factors of M1/M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal 11:29
Wynn TA, Barron L (2010) Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 30(3):245–257
Klingberg F et al (2018) The ED-A domain enhances the capacity of fibronectin to store latent TGF-β binding protein-1 in the fibroblast matrix. J Cell Sci. https://doi.org/10.1242/jcs.201293
Klingberg F et al (2018) The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix. J Cell Sci 131:jcs201293
Reiter RJ (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev 12:151–180
Patel PD, Pontrello C, Burke S (2004) Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry 55:428–433
Côté F et al (2003) Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci 100:13525–13530
Walther DJ, Bader M (2003) A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 66:1673–1680
Bertrand PP, Bertrand RL (2010) Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci Basic Clin 153:47–57
Imai S-I (2009) The NAD World: a new systemic regulatory network for metabolism and aging—Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys 53:65
Badawy AA-B (2015) Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep 35:e00261
Pfefferkorn ER, Eckel M, Rebhun S (1986) Interferon-γ suppresses the growth of Toxoplasma gondii in human fibroblasts through starvation for tryptophan. Mol Biochem Parasitol 20:215–224
Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL (1998) Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281:1191–1193
Badawy AA-B, Namboodiri AM, Moffett JR (2016) The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin Sci 130:1327–1333
Roman AC et al (2018) The aryl hydrocarbon receptor in the crossroad of signalling networks with therapeutic value. Pharmacol Ther 185:50–63
Dere E, Lo R, Celius T, Matthews J, Zacharewski TR (2011) Integration of genome-wide computation DRE search, AhR ChIP-chip and gene expression analyses of TCDD-elicited responses in the mouse liver. BMC Genom 12:365
Nguyen LP, Bradfield CA (2007) The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 21:102–116
Flaveny CA, Murray IA, Perdew GH (2009) Differential gene regulation by the human and mouse aryl hydrocarbon receptor. Toxicol Sci 114:217–225
Flaveny C, Reen RK, Kusnadi A, Perdew GH (2008) The mouse and human Ah receptor differ in recognition of LXXLL motifs. Arch Biochem Biophys 471:215–223
Beischlag TV et al (2008) The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukar Gene Expr 18(3):207–250
Opitz CA et al (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197–203
DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH (2010) Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci 115:89–97
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld J-C, Stockinger B (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453:106
Henry E, Bemis J, Henry O, Kende A, Gasiewicz T (2006) A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys 450:67–77
Lowe MM et al (2014) Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS One 9:e87877
Poormasjedi-Meibod M-SS, Hartwell R, Kilani RT, Ghahary A (2014) Anti-scarring properties of different tryptophan derivatives. PloS one 9:e91955
Poormasjedi-Meibod MS, Salimi Elizei S, Leung V, Baradar Jalili R, Ko F, Ghahary A (2016) Kynurenine modulates MMP-1 and type-I collagen expression via aryl hydrocarbon receptor activation in dermal fibroblasts. J Cell Physiol 231:2749–2760
Poormasjedi-Meibod MS, Pakyari M, Jackson JK, Salimi Elizei S, Ghahary A (2016) Development of a nanofibrous wound dressing with an antifibrogenic properties in vitro and in vivo model. J Biomed Mater Res Part A 104:2334–2344
Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR (1991) Blood–brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 56:2007–2017
Parrott J, Redus L, Santana-Coelho D, Morales J, Gao X, O’connor J (2016) Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 6:e918
Meier TB et al (2016) Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun 53:39–48
Birner A et al (2017) Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS One 12:e0172699
Lovelace MD et al (2016) Current evidence for a role of the kynurenine pathway of tryptophan metabolism in multiple sclerosis. Front Immunol 7:246
Chavez-Munoz C et al (2012) Application of an Indoleamine 2, 3-dioxygenase–expressing skin substitute improves scar formation in a fibrotic animal model. J Investig Dermatol 132:1501–1505
Hartwell R, Poormasjedi-Meibod MS, Chavez-Munoz C, Jalili RB, Hossenini-Tabatabaei A, Ghahary A (2015) An in-situ forming skin substitute improves healing outcome in a hypertrophic scar model. Tissue Eng Part A. 18(21):1085–1094
Liu H, Liu L, Fletcher BS, Visner GA (2006) Sleeping beauty-based gene therapy with indoleamine 2, 3-dioxygenase inhibits lung allograft fibrosis. FASEB J 20:2384–2386
Li Y, Kilani RT, Rahmani-Neishaboor E, Jalili RB, Ghahary A (2014) Kynurenine increases matrix metalloproteinase-1 and -3 expression in cultured dermal fibroblasts and improves scarring in vivo. J Investig Dermatol 134:643–650
Yu H et al (2014) The aryl hydrocarbon receptor suppresses osteoblast proliferation and differentiation through the activation of the ERK signaling pathway. Toxicol Appl Pharmacol 280:502–510
Ye M et al (2018) Activation of the aryl hydrocarbon receptor leads to resistance to EGFR TKIs in non-small cell lung cancer by activating src-mediated bypass signaling. Clin Cancer Res 24:1227–1239
Borlak J, Jenke HS (2008) Cross-talk between aryl hydrocarbon receptor and mitogen-activated protein kinase signaling pathway in liver cancer through c-raf transcriptional regulation. Mol Cancer Res 6:1326–1336
Aguilera-Montilla N et al (2013) Aryl hydrocarbon receptor contributes to the MEK/ERK-dependent maintenance of the immature state of human dendritic cells. Blood 121:e108–e117
Li D et al (2012) Effects of indoleamine 2, 3-dioxygenases in carbon tetrachloride-induced hepatitis model of rats. Cell Biochem Funct 30:309–314
Ogiso H et al (2016) The deficiency of indoleamine 2, 3-dioxygenase aggravates the CCl4-induced liver fibrosis in mice. PLoS One 11:e0162183
Giri SN, Hyde DM, Marafino BJ Jr (1986) Ameliorating effect of murine interferon gamma on bleomycin-induced lung collagen fibrosis in mice. Biochem Med Metab Biol 36:194–197
Gurujeyalakshmi G, Giri S (1995) Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-β and procollagen I and III gene expression. Exp Lung Res 21:791–808
Baroni GS, D’Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A (1996) Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology 23:1189–1199
Weng HL, Cai WM, Liu RH (2001) Animal experiment and clinical study of effect of gamma-interferon on hepatic fibrosis. World J Gastroenterol 7:42
Lupher ML Jr, Gallatin WM (2006) Regulation of fibrosis by the immune system. Adv Immunol 89:245–288
Young HA, Hardy KJ (1995) Role of interferon-γ in immune cell regulation. J Leukoc Biol 58:373–381
Low S, Kitada S, Lee D (1991) Interferon-gamma inhibits collagen synthesis by human Tenon’s capsule fibroblasts in vitro. Investig Ophthalmol Vis Sci 32:2964–2969
Clark JG, Dedon T, Wayner E, Carter W (1989) Effects of interferon-gamma on expression of cell surface receptors for collagen and deposition of newly synthesized collagen by cultured human lung fibroblasts. J Clin Investig 83:1505–1511
Ghosh AK, Bhattacharyya S, Mori Y, Varga J (2006) Inhibition of collagen gene expression by interferon-γ: novel role of the CCAAT/enhancer binding protein β (C/EBPβ). J Cell Physiol 207:251–260
Eickelberg O et al (2001) Molecular mechanisms of TGF-β antagonism by interferon γ and cyclosporine A in lung fibroblasts. FASEB J 15:797–806
Ulloa L, Doody J, Massagué J (1999) Inhibition of transforming growth factor-β/SMAD signalling by the interferon-γ/STAT pathway. Nature 397:710
Amento EP, Ehsani N, Palmer H, Libby P (1991) Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 11:1223–1230
Rezzonico R, Burger D, Dayer J-M (1998) Direct contact between T lymphocytes and human dermal fibroblasts or synoviocytes down-regulates types I and III collagen production via cell-associated cytokines. J Biol Chem 273:18720–18728
Dai W, Gupta SL (1990) Regulation of indoleamine 2, 3-dioxygenase gene expression in human fibroblasts by interferon-gamma Upstream control region discriminates between interferon. J Biol Chem 265:19871–19877
Yadav MC, Burudi E, Alirezaei M, Flynn CC, Watry DD, Lanigan CM, Fox HS (2007) IFN-γ-induced IDO and WRS expression in microglia is differentially regulated by IL-4. Glia 55:1385–1396
Malone D, Dolan P, Brown R, Kalayoglu M, Arend R, Byrne G, Ozaki Y (1994) Interferon gamma induced production of indoleamine 2, 3 dioxygenase in cultured human synovial cells. J Rheumatol 21:1011–1019
MacKenzie C et al (1999) Cytokine mediated regulation of interferon-gamma-induced IDO activation. Tryptophan, serotonin, and melatonin. Springer, Boston, MA, pp 533–539
Jung ID, Lee C-M, Jeong Y-I, Lee JS, Park WS, Han J, Park Y-M (2007) Differential regulation of indoleamine 2, 3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Lett 581:1449–1456
Sarkar SA, Wong R, Hackl SI, Moua O, Gill RG, Wiseman A, Davidson HW, Hutton JC (2007) Induction of indoleamine 2, 3-dioxygenase by interferon-γ in human islets. Diabetes 56:72–79
Mittal D et al (2013) Indoleamine 2, 3-dioxygenase activity contributes to local immune suppression in the skin expressing human papillomavirus oncoprotein e7. J Investig Dermatol 133:2686–2694
Sarkhosh K, Tredget EE, Karami A, Uludag H, Iwashina T, Kilani RT, Ghahary A (2003) Immune cell proliferation is suppressed by the interferon-γ-induced indoleamine 2, 3-dioxygenase expression of fibroblasts populated in collagen gel (FPCG). J Cell Biochem 90:206–217
Ghahary A, Li Y, Tredget EE, Kilani RT, Iwashina T, Karami A, Lin X (2004) Expression of indoleamine 2, 3-dioxygenase in dermal fibroblasts functions as a local immunosuppressive factor. J Investig Dermatol 122:953–964
Li Y, Tredget EE, Ghaffari A, Lin X, Kilani RT, Ghahary A (2006) Local expression of indoleamine 2, 3-dioxygenase protects engraftment of xenogeneic skin substitute. J Investig Dermatol 126:128–136
Fernandez-Salguero P et al (1995) Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268:722–726
Peterson TC, Hodgson P, Fernandez-Salguero P, Neumeister M, Gonzalez FJ (2000) Hepatic fibrosis and cytochrome P450: experimental models of fibrosis compared to AHR knockout mice. Hepatol Res 17:112–125
Corchero J, Martín-Partido G, Dallas SL, Fernández-Salguero PM (2004) Liver portal fibrosis in dioxin receptor-null mice that overexpress the latent transforming growth factor-β-binding protein-1. Int J Exp Pathol 85:295–302
Hemsworth-Peterson T (2013) Role of JNK signalling and ahr in fibrosis, implications for new therapeutics. Pancreat Disord Ther 3:2
Monteleone I et al (2016) Aryl hydrocarbon receptor-driven signals inhibit collagen synthesis in the gut. Eur J Immunol 46:1047–1057
Woeller CF, Roztocil E, Hammond CL, Feldon SE, Phipps RP (2016) The aryl hydrocarbon receptor and its ligands inhibit myofibroblast formation and activation: implications for thyroid eye disease. Am J Pathol 186:3189–3202
Lehmann GM et al (2011) The aryl hydrocarbon receptor ligand ITE inhibits TGFβ1-induced human myofibroblast differentiation. Am J Pathol 178:1556–1567
Murai M, Tsuji G, Hashimoto-Hachiya A, Kawakami Y, Furue M, Mitoma C (2018) An endogenous tryptophan photo-product, FICZ, is potentially involved in photo-aging by reducing TGF-β-regulated collagen homeostasis. J Dermatol Sci 89:19–26
Wrighton KH, Lin X, Feng X-H (2009) Phospho-control of TGF-β superfamily signaling. Cell Res 19:8
Wang G, Matsuura I, He D, Liu F (2009) Transforming growth factor-β-inducible phosphorylation of Smad3. J Biol Chem 284:9663–9673
Hough C, Radu M, Doré JJ (2012) Tgf-beta induced Erk phosphorylation of smad linker region regulates smad signaling. PLoS One 7:e42513
Hayashida T, Decaestecker M, Schnaper HW (2003) Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-β-dependent responses in human mesangial cells. FASEB J 17:1576–1578
Engel ME, McDonnell MA, Law BK, Moses HL (1999) Interdependent SMAD and JNK signaling in transforming growth factor-β-mediated transcription. J Biol Chem 274:37413–37420
Mori S et al (2004) TGF-β and HGF transmit the signals through JNK-dependent Smad2/3 phosphorylation at the linker regions. Oncogene 23:7416
Alarcón C et al (2009) Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell 139:757–769
Kamaraju AK, Roberts AB (2005) Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-β-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem 280:1024–1036
Rostam MA, Kamato D, Piva TJ, Zheng W, Little PJ, Osman N (2016) The role of specific Smad linker region phosphorylation in TGF-β mediated expression of glycosaminoglycan synthesizing enzymes in vascular smooth muscle. Cell Signal 28:956–966
Nishida M, Okumura Y, Sato H, Hamaoka K (2008) Delayed inhibition of p38 mitogen-activated protein kinase ameliorates renal fibrosis in obstructive nephropathy. Nephrol Dial Transplant 23:2520–2524
Stambe C, Atkins RC, Tesch GH, Masaki T, Schreiner GF, Nikolic-Paterson DJ (2004) The role of p38α mitogen-activated protein kinase activation in renal fibrosis. J Am Soc Nephrol 15:370–379
Akhmetshina A et al (2012) Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun 3:735
Xu L et al (2017) Activation of Wnt/β-catenin signalling is required for TGF-β/Smad2/3 signalling during myofibroblast proliferation. J Cell Mol Med 21:1545–1554
Baarsma HA et al (2011) Activation of WNT/β-catenin signaling in pulmonary fibroblasts by TGF-β1 is increased in chronic obstructive pulmonary disease. PLoS One 6:e25450
Ihn H, Yamane K, Tamaki K (2005) Increased phosphorylation and activation of mitogen-activated protein kinase p38 in scleroderma fibroblasts. J Gen Intern Med 20:247–255
Dolivo D, Larson S, Dominko T (2017) FGF2-mediated attenuation of myofibroblast activation is modulated by distinct MAPK signaling pathways in human dermal fibroblasts. J Dermatol Sci 88:339–348
Molkentin JD et al (2017) Fibroblast-specific genetic manipulation of p38 MAPK in vivo reveals its central regulatory role in fibrosis. Circulation CIRCULATIONAHA 116:026238
Choi SY et al (2016) Piceatannol attenuates renal fibrosis induced by unilateral ureteral obstruction via downregulation of histone deacetylase 4/5 or p38-MAPK signaling. PLoS One 11:e0167340
Sugiyama N, Kohno M, Yokoyama T (2011) Inhibition of the p38 MAPK pathway ameliorates renal fibrosis in an NPHP2 mouse model. Nephrol Dial Transplant 27(4):1351–1358
See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, Itescu S, Krum H (2004) p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol 44:1679–1689
Matysik-Woźniak A, Paduch R, Turski WA, Maciejewski R, Jünemann AG, Rejdak R (2017) Effects of tryptophan, kynurenine and kynurenic acid exerted on human reconstructed corneal epithelium in vitro. Pharmacol Rep 69:722–729
Morita T et al (1999) l-tryptophan-kynurenine pathway metabolite 3-hydroxyanthranilic acid induces apoptosis in macrophage-derived cells under pathophysiological conditions, tryptophan, serotonin, and melatonin. Springer, Boston, MA, pp 559–563
Poormasjedi-Meibod M-S, Jalili RB, Hosseini-Tabatabaei A, Hartwell R, Ghahary A (2013) Immuno-regulatory function of indoleamine 2, 3 dioxygenase through modulation of innate immune responses. PLoS One 8:e71044
Fallarino F et al (2003) T cell apoptosis by kynurenines. Developments in tryptophan and serotonin metabolism. Springer, Boston, MA, pp 183–190
Dagenais-Lussier X, Aounallah M, Mehraj V, El-Far M, Tremblay C, Sekaly R-P, Routy J-P, Van Grevenynghe J (2016) Kynurenine reduces memory CD4 T-cell survival by interfering with interleukin-2 signaling early during HIV-1 infection. J Virol 90:7967–7979
Belladonna ML et al (2006) Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol 177:130–137
Terness P, Bauer TM, Röse L, Dufter C, Watzlik A, Simon H, Opelz G (2002) Inhibition of allogeneic T cell proliferation by indoleamine 2, 3-dioxygenase–expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 196:447–457
Morita T et al (2001) 3-Hydroxyanthranilic acid, an l-tryptophan metabolite, induces apoptosis in monocyte-derived cells stimulated by interferon-γ. Ann Clin Biochem 38:242–251
Khalil N, Corne S, Whitman C, Yacyshyn H (1996) Plasmin regulates the activation of cell-associated latent TGF-beta 1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am J Respir Cell Mol Biol 15:252–259
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Investig 101:890–898
Song E, Ouyang N, Hörbelt M, Antus B, Wang M, Exton MS (2000) Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol 204:19–28
Duffield JS et al (2005) Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Investig 115:56–65
Wynn TA (2004) Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol 4:583
Mann DA, Oakley F (2013) Serotonin paracrine signaling in tissue fibrosis. Biochimica et Biophysica Acta (BBA) Mol Basis Dis 1832:905–910
Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, Metcalfe DD (2006) 5-Hydroxytryptamine induces mast cell adhesion and migration. J Immunol 177:6422–6432
Boehme SA, Lio FM, Sikora L, Pandit TS, Lavrador K, Rao SP, Sriramarao P (2004) Cutting edge: serotonin is a chemotactic factor for eosinophils and functions additively with eotaxin. J Immunol 173:3599–3603
Li N, Ghia J-E, Wang H, McClemens J, Cote F, Suehiro Y, Mallet J, Khan WI (2011) Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol 178:662–671
Müller T et al (2009) 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One 4:e6453
Idzko M et al (2004) The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol 172:6011–6019
Soga F, Katoh N, Inoue T, Kishimoto S (2007) Serotonin activates human monocytes and prevents apoptosis. J Investig Dermatol 127:1947–1955
Dürk T et al (2005) 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int Immunol 17:599–606
Rosenberg T, Lattimer R, Montgomery P, Wiens C, Levy L (2017) The relationship of ssrI and snrI usage with interstitial lung disease and bronchiectasis in an elderly population: a case–control study. Clin Interv Aging 12:1977
Thornton C, Maher TM, Hansell D, Nicholson AG, Wells AU (2009) Pulmonary fibrosis associated with psychotropic drug therapy: a case report. J Med Case Rep 3:126
Beretta L, Cossu M, Marchini M, Cappiello F, Artoni A, Motta G, Scorza R (2008) A polymorphism in the human serotonin 5-HT 2A receptor gene may protect against systemic sclerosis by reducing platelet aggregation. Arthritis Res Ther 10:R103
Beretta L, Scorza R (2009) 5HT 2A polymorphism His452Tyr in a German Caucasian systemic sclerosis population–authors’ response. Arthritis Res Ther 11:404
Kirsten H, Burkhardt J, Hantmann H, Hunzelmann N, Vaith P, Ahnert P, Melchers I (2009) 5HT 2A polymorphism His452Tyr in a German Caucasian systemic sclerosis population. Arthritis Res Ther 11:403
Hazelwood LA, Sanders-Bush E (2004) His452Tyr polymorphism in the human 5-HT2A receptor destabilizes the signaling conformation. Mol Pharmacol 66:1293–1300
Asselin J, Gibbins JM, Achison M, Lee YH, Morton LF, Farndale RW, Barnes MJ, Watson SP (1997) A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase Cγ2 in platelets independent of the integrin α2β1. Blood 89:1235–1242
Blake RA, Schieven GL, Watson SP (1994) Collagen stimulates tyrosine phosphorylation of phospholipase C-γ2 but not phospholipase C-γ1 in human platelets. FEBS Lett 353:212–216
Mackenzie LS, Lymn JS, Hughes AD (2013) Linking phospholipase C isoforms with differentiation function in human vascular smooth muscle cells. Biochimica et Biophysica Acta (BBA) Mol Cell Res 1833:3006–3012
Zhu X et al (2017) Phospholipase Cε deficiency delays the early stage of cutaneous wound healing and attenuates scar formation in mice. Biochem Biophys Res Commun 484:144–151
Mekontso-Dessap A et al (2006) Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation 113:81–89
Gustafsson BI et al (2005) Long-term serotonin administration induces heart valve disease in rats. Circulation 111:1517–1522
Dees C et al (2011) Platelet-derived serotonin links vascular disease and tissue fibrosis. J Exp Med 208:961–972 (jem. 20101629)
Königshoff M et al (2010) Increased expression of 5-hydroxytryptamine2A/B receptors in idiopathic pulmonary fibrosis: a rationale for therapeutic intervention. Thorax thx. 2009:134353
Janssen W et al (2015) 5-HT2B receptor antagonists inhibit fibrosis and protect from RV heart failure. BioMed Res Int 2015:1–8
Elaidy SM, Essawy SS (2016) The antifibrotic effects of alveolar macrophages 5-HT2C receptors blockade on bleomycin-induced pulmonary fibrosis in rats. Pharmacol Rep 68:1244–1253
Tawfik MK, Makary S (2017) 5-HT7 receptor antagonism (SB-269970) attenuates bleomycin-induced pulmonary fibrosis in rats via downregulating oxidative burden and inflammatory cascades and ameliorating collagen deposition: comparison to terguride. Eur J Pharmacol 814:114–123
Löfdahl A et al (2016) 5‐HT2B receptor antagonists attenuate myofibroblast differentiation and subsequent fibrotic responses in vitro and in vivo. Physiol Rep 4
Jaffré F, Callebert J, Sarre A, Etienne N, Nebigil CG, Launay J-M, Maroteaux L, Monassier L (2004) Involvement of the serotonin 5-HT2B receptor in cardiac hypertrophy linked to sympathetic stimulation: control of interleukin-6, interleukin-1β, and tumor necrosis factor-α cytokine production by ventricular fibroblasts. Circulation 110:969–974
Frey N, Olson E (2003) Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 65:45–79
Jaffré F et al (2009) Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ Res 104:113–123
Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, Mann DA (2006) A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol 169:861–876
Yabanoglu S, Akkiki M, Seguelas M-H, Mialet-Perez J, Parini A, Pizzinat N (2009) Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J Mol Cell Cardiol 46:518–525
Chen C et al (2014) Serotonin drives the activation of pulmonary artery adventitial fibroblasts and TGF-β1/Smad3-mediated fibrotic responses through 5-HT2A receptors. Mol Cell Biochem 397:267–276
Moreno AC, Clara RO, Coimbra JB, Júlio AR (2013) The expanding roles of 1-methyl-tryptophan (1-MT): in addition to inhibiting kynurenine production, 1-MT activates the synthesis of melatonin in skin cells. FEBS J 280(19):4782–4792
Li Y, Hu N, Yang D, Oxenkrug G, Yang Q (2017) Regulating the balance between the kynurenine and serotonin pathways of tryptophan metabolism. FEBS J 284:948–966
Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J (2003) Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol 196:144–153
Slominski A et al (2002) Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J 16:896–898
Sheipouri D, Grant R, Bustamante S, Lovejoy D, Guillemin GJ, Braidy N (2015) Characterisation of the kynurenine pathway in skin-derived fibroblasts and keratinocytes. J Cell Biochem 116:903–922
Sheipouri D, Braidy N, Guillemin GJ (2012) Kynurenine pathway in skin cells: Implications for UV-induced skin damage. Int J Tryptophan Res 5:IJTR. S9835
Papp A, Hartwell R, Evans M, Ghahary A (2018) The safety and tolerability of topically delivered kynurenic acid in humans. A phase 1 randomized double-blind clinical trial. J Pharm Sci 107:1572–1576
BirchBioMed (2018) Birchbiomed is cleared to begin first-of-its-kind phase II clinical trial for ground-breaking anti-scarring drug. In: Elliott S (eds) BirchBioMed, Vancouver, BC, pp 1–3
Eickelberg O, Pansky A, Mussmann R, Bihl M, Tamm M, Hildebrand P, Perruchoud AP, Roth M (1999) Transforming growth factor-β1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J Biol Chem 274:12933–12938
Yao Z et al (2010) TGF-β IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci 107:15535–15540
Elias J, Lentz V, Cummings P (1991) Transforming growth factor-beta regulation of IL-6 production by unstimulated and IL-1-stimulated human fibroblasts. J Immunol 146:3437–3443
Seong GJ, Hong S, Jung S-A, Lee J-J, Lim E, Kim S-J, Lee JH (2009) TGF-β-induced interleukin-6 participates in transdifferentiation of human Tenon’s fibroblasts to myofibroblasts. Mol Vis 15:2123
Funding
This work was funded by a National Institutes of Health Award (NIH R01GM85456) to Tanja Dominko and a National Science Foundation Integrative Graduate Education and Research Traineeship (Grant number DGE 1144804) awarded to David Dolivo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Dolivo, D.M., Larson, S.A. & Dominko, T. Tryptophan metabolites kynurenine and serotonin regulate fibroblast activation and fibrosis. Cell. Mol. Life Sci. 75, 3663–3681 (2018). https://doi.org/10.1007/s00018-018-2880-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2880-2