Abstract
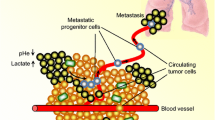
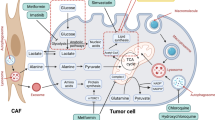
Pancreatic ductal adenocarcinoma is prone to distant metastasis and is expected to become the second leading cause of cancer-related death. In an extremely nutrient-deficient and hypoxic environment resulting from uncontrolled growth, vascular disturbances and desmoplastic reactions, pancreatic cancer cells utilize “metabolic reprogramming” to satisfy their energy demand and support malignant behaviors such as metastasis. Notably, pancreatic cancer cells show extensive enhancement of glycolysis, including glycolytic enzyme overexpression and increased lactate production, and this is caused by mitochondrial dysfunction, cancer driver genes, specific transcription factors, a hypoxic tumor microenvironment and stromal cells, such as cancer-associated fibroblasts and tumor-associated macrophages. The metabolic switch from oxidative phosphorylation to glycolysis in pancreatic cancer cells regulates the invasion–metastasis cascade by promoting epithelial–mesenchymal transition, tumor angiogenesis and the metastatic colonization of distant organs. In addition to aerobic glycolysis, oxidative phosphorylation also plays a critical role in pancreatic cancer metastasis in ways that remain unclear. In this review, we expound on the intracellular and extracellular causes of the enhancement of glycolysis in pancreatic cancer and the strong association between glycolysis and cancer metastasis, which we expect will yield new therapeutic approaches targeting cancer metabolism.



Similar content being viewed by others
Abbreviations
- PDAC:
-
Pancreatic ductal adenocarcinoma
- EMT:
-
Epithelial–mesenchymal transition
- CAFs:
-
Cancer-associated fibroblasts
- OXPHOS:
-
Oxidative phosphorylation
- NOX:
-
NADPH oxidase
- mtDNA:
-
Mitochondrial DNA
- ΔΨm :
-
Membrane potential
- PFK:
-
Phosphofructokinase
- ALDOA:
-
Aldolase A
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- PGM:
-
Phosphoglycerate mutase
- ENO:
-
Enolase
- PKM:
-
Pyruvate kinase muscle isozyme
- LDHA:
-
Lactate dehydrogenase A
- TME:
-
Tumor microenvironment
- TGF:
-
Transforming growth factor
- IGF1R:
-
Insulin-like growth factor 1 receptor
- MAPK:
-
Mitogen-activated protein kinase
- PI3K/AKT:
-
Phosphoinositide 3-kinase/protein kinase B
- mTOR:
-
Mammalian target of rapamycin
- NF-kB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PDLCs:
-
Patient-derived pancreatic cancer cell lines
- PGC-1α:
-
Peroxisome proliferator-activated receptor γ coactivator-1α
- SDH:
-
Succinate dehydrogenase
- HK:
-
Hexokinase
- TCA:
-
Tricarboxylic acid cycle
- HIF:
-
Hypoxia-induced factor
- PPP:
-
Pentose phosphate pathway
- GOF mutp53:
-
Gain-of-function of mutant p53
- GLUT:
-
Glucose transporter
- AMPK:
-
AMP-activated protein kinase
- TIGAR:
-
Tp53-induced glycolysis and apoptosis regulator
- GAMT:
-
Guanidinoacetate N-methyltransferase
- GLS2:
-
Glutaminase-2
- ROS:
-
Reactive oxygen species
- PFKFB:
-
6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase
- ACL:
-
ATP citrate lyase
- PDK:
-
Pyruvate dehydrogenase kinase
- PGI/AMF:
-
Glucose-6-phosphate isomerase/Autocrine motility factor
- VEGF:
-
Vascular epidermal growth factor
- TGFBI:
-
Transforming growth factor beta-induced
- FAK:
-
Focal adhesion kinase
- ECM:
-
Extracellular matrix
- EMT-TFs:
-
EMT-TF
- MMP:
-
Matrix metalloproteinase
- 6-PGD:
-
6-Phosphogluconate dehydrogenase
- FGF:
-
Fibroblast growth factor
- GPR81:
-
G-protein-coupled receptors 81
- MCT:
-
Monocarboxylate transporters
- TAMs:
-
Tumor-associated macrophages
- CXCL:
-
CXC chemokine ligand
- CXCR:
-
CXC chemokine receptor
- ECs:
-
Endothelial cells
- CTCs:
-
Circulating tumor cells
- CSCs:
-
Cancer stem cells
- HSCs:
-
Hepatic stellate cells
- HMFs:
-
Hepatic myofibroblasts
- GOT:
-
Glutamic-oxaloacetic transaminase
References
Kamisawa T, Wood LD, Itoi T, Takaori K (2016) Pancreatic cancer. Lancet 388:73–85. https://doi.org/10.1016/S0140-6736(16)00141-0
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Rahib L et al (2014) Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74:2913. http://cancerres.aacrjournals.org/content/74/11/2913.abstract. Accessed 17 Aug 2019
Poruk KE, Firpo MA, Adler DG, Mulvihill SJ (2013) Screening for pancreatic cancer: why, how, and who? Ann Surg 257:17–26
Giovannetti E et al (2017) Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin Cancer Biol 44:43–59
Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134:703–707
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7:11–20
Semenza GL et al (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271:32529–32537
Robertson-Tessi M, Gillies RJ, Gatenby RA, Anderson ARA (2015) Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Res 75:1567. http://cancerres.aacrjournals.org/content/75/8/1567.abstract. Accessed 17 Aug 2019
Jia D et al (2019) Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci USA 116:3909–3918
Sancho P et al (2015) MYC/PGC-1alpha balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab 22:590–605
Yang Y et al (2019) MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun 10:809. https://doi.org/10.1038/s41467-019-08759-0
Sousa CM, Kimmelman AC (2014) The complex landscape of pancreatic cancer metabolism. Carcinogenesis 35:1441–1450
McDonald OG et al (2017) Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet 49:367–376
Tsutsumi S, Yanagawa T, Shimura T, Kuwano H, Raz A (2004) Autocrine motility factor signaling enhances pancreatic cancer metastasis. Clin Cancer Res 10:7775–7784
Azoitei N et al (2016) PKM2 promotes tumor angiogenesis by regulating HIF-1alpha through NF-kappaB activation. Mol Cancer 15:3
Zhao H et al (2017) Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J Cell Mol Med 21:2055–2067
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Warburg O (1956) On respiratory impairment in cancer cells. Science 124:269–270
Jia D, Park JH, Jung KH, Levine H, Kaipparettu BA (2018) Elucidating the metabolic plasticity of cancer: mitochondrial reprogramming and hybrid metabolic states. Cells 7:21
Guha M, Avadhani NG (2013) Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion 13:577–591
Ishikawa K et al (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320:661–664
Jones JB et al (2001) Detection of mitochondrial DNA mutations in pancreatic cancer offers a “Mass”-ive advantage over detection of nuclear DNA mutations. Cancer Res 61:1299
Hardie RA et al (2017) Mitochondrial mutations and metabolic adaptation in pancreatic cancer. Cancer Metab 5:2
Donadelli M, Dando I, Dalla Pozza E, Palmieri M (2015) Mitochondrial uncoupling protein 2 and pancreatic cancer: a new potential target therapy. World J Gastroenterol 21:3232–3238
Lu W et al (2012) Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol 10:e1001326
Hanahan D, Weinberg Robert A (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Jones S et al (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321:1801–1806
Yun J et al (2009) Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325:1555–1559
Ying H et al (2012) Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149:656–670
Halbrook CJ, Lyssiotis CA (2017) Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell 31:5–19
He TL et al (2015) The c-Myc-LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer. Med Oncol 32:187
Ji S et al (2016) FBW7 (F-box and WD Repeat Domain-Containing 7) negatively regulates glucose metabolism by targeting the c-Myc/TXNIP (Thioredoxin-Binding Protein) axis in pancreatic cancer. Clin Cancer Res 22:3950–3960
Zubair H et al (2016) Glucose metabolism reprogrammed by overexpression of IKKepsilon promotes pancreatic tumor growth. Cancer Res 76:7254–7264
Butera G et al (2018) Mutant p53 prevents GAPDH nuclear translocation in pancreatic cancer cells favoring glycolysis and 2-deoxyglucose sensitivity. BBA-Mol Cell Res 1865:1914–1923
Zhang C et al (2013) Tumour-associated mutant p53 drives the Warburg effect. Nat Commun 4:2935. https://doi.org/10.1038/ncomms3935
Rajeshkumar NV et al (2015) Therapeutic targeting of the warburg effect in pancreatic cancer relies on an absence of p53 function. Cancer Res 75:3355–3364
Buchakjian MR, Kornbluth S (2010) The engine driving the ship: metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol 11:715–727
Bensaad K et al (2006) TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126:107–120
Kotiah SD, Caro J (2010) Elevation of PFKFB3 and TIGAR levels in pancreatic cancer. J Clin Oncol 28
Ros S et al (2017) 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 is essential for p53-null cancer cells. Oncogene 36:3287. https://doi.org/10.1038/onc.2016.477
Nagarajan A et al (2017) Paraoxonase 2 facilitates pancreatic cancer growth and metastasis by stimulating GLUT1-Mediated glucose transport. Mol Cell 67:685–701.e6
Altenberg B, Greulich KO (2004) Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 84:1014–1020
King A, Selak MA, Gottlieb E (2006) Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene 25:4675–4682
Wellen KE et al (2009) ATP-citrate lyase links cellular metabolism to histone acetylation. Ann NY Acad Sci 324:1076-1080. https://www.ncbi.nlm.nih.gov/pubmed/19461003. Accessed 17 Aug 2019
Cui J et al (2014) FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res 20:2595–2606
Hua S et al (2018) miR-139-5p inhibits aerobic glycolysis, cell proliferation, migration, and invasion in hepatocellular carcinoma via a reciprocal regulatory interaction with ETS1. Oncogene 37:1624–1636
Yamamoto T et al (2014) Reduced methylation of PFKFB3 in cancer cells shunts glucose towards the pentose phosphate pathway. Nat Commun 5:3480
Li FL et al (2018) Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat Commun 9:508
Zhao D et al (2013) Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell 23:464–476
Neesse A et al (2019) Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut 68:159–171
Yeung SJ, Pan J, Lee MH (2008) Roles of p53, MYC and HIF-1 in regulating glycolysis—the seventh hallmark of cancer. Cell Mol Life Sci 65:3981–3999
Zhang Q et al (2017) Hypoxia-inducible factor-2alpha promotes tumor progression and has crosstalk with Wnt/beta-catenin signaling in pancreatic cancer. Mol Cancer 16:119
He G, Jiang Y, Zhang B, Wu G (2014) The effect of HIF-1alpha on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac J Clin Nutr 23:174–180
Yoon DY et al (2001) Identification of genes differentially induced by hypoxia in pancreatic cancer cells. Biochem Biophys Res Commun 288:882–886
Clem BF et al (2013) Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther 12:1461–1470
Bobarykina AY et al (2006) Hypoxic regulation of PFKFB-3 and PFKFB-4 gene expression in gastric and pancreatic cancer cell lines and expression of PFKFB genes in gastric cancers. Acta Biochim Pol 53:789–799
Zhang H et al (2016) HIF-1alpha activates hypoxia-induced PFKFB4 expression in human bladder cancer cells. Biochem Biophys Res Commun 476:146–152
Goidts V et al (2012) RNAi screening in glioma stem-like cells identifies PFKFB4 as a key molecule important for cancer cell survival. Oncogene 31:3235–3243
Minchenko OH, Tsuchihara K, Minchenko DO, Bikfalvi A, Esumi H (2014) Mechanisms of regulation of PFKFB expression in pancreatic and gastric cancer cells. World J Gastroenterol 20:13705–13717
Cheung EC, Ludwig RL, Vousden KH (2012) Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci USA 109:20491–20496
Ye H et al (2018) Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis 9:453
Yan B et al (2018) Paracrine HGF/c-MET enhances the stem cell-like potential and glycolysis of pancreatic cancer cells via activation of YAP/HIF-1alpha. Exp Cell Res 371:63–71
Shi S et al (2016) VEGF promotes glycolysis in pancreatic cancer via HIF1alpha up-regulation. Curr Mol Med 16:394–403
Costanza B et al (2019) Transforming growth factor beta-induced, an extracellular matrix interacting protein, enhances glycolysis and promotes pancreatic cancer cell migration. Int J Cancer 145:1570–1584
Ishida S, Andreux P, Poitry-Yamate C, Auwerx J, Hanahan D (2013) Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci USA 110:19507–19512
Wilde L et al (2017) Metabolic coupling and the Reverse Warburg Effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol 44:198–203
Chen S et al (2018) MiR-21-mediated metabolic alteration of cancer-associated fibroblasts and its effect on pancreatic cancer cell behavior. Int J Biol Sci 14:100–110
Shan T et al (2017) Cancer-associated fibroblasts enhance pancreatic cancer cell invasion by remodeling the metabolic conversion mechanism. Oncol Rep 37:1971–1979
Weissmueller S et al (2014) Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell 157:382–394
Roe JS et al (2017) Enhancer reprogramming promotes pancreatic cancer metastasis. Cell 170:875–888.e20
Deng SJ et al (2018) Hypoxia-induced LncRNA-BX111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription. Oncogene 37:5811–5828
Li Z et al (2018) Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene 37:3822–3838. https://doi.org/10.1038/s41388-018-0237-9
Gao H et al (2016) Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell 166:47–62
Roland CL et al (2014) Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res 74:5301–5310
Dovmark TH, Saccomano M, Hulikova A, Alves F, Swietach P (2017) Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene 36:4538. https://doi.org/10.1038/onc.2017.71
Lambert AW, Pattabiraman DR, Weinberg RA (2017) Emerging biological principles of metastasis. Cell 168:670–691. https://doi.org/10.1016/j.cell.2016.11.037
Zheng X et al (2015) Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527:525–530
Krebs AM et al (2017) The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol 19:518–529
Daemen A et al (2015) Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc Natl Acad Sci USA 112:E4410–E4417
Payen VL, Porporato PE, Baselet B, Sonveaux P (2016) Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci 73:1333–1348
Yalcin A et al (2017) 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase-3 is required for transforming growth factor beta1-enhanced invasion of Panc1 cells in vitro. Biochem Biophys Res Commun 484:687–693
Li H-M et al (2017) Blockage of glycolysis by targeting PFKFB3 suppresses tumor growth and metastasis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res 36:7
Ji S et al (2016) ALDOA functions as an oncogene in the highly metastatic pancreatic cancer. Cancer Lett 374:127–135
Baumann F et al (2009) Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol 11:368–380
Guo Q (2017) Changes in mitochondrial function during EMT induced by TGFbeta-1 in pancreatic cancer. Oncol Lett 13:1575–1580
Principe M et al (2017) Alpha-enolase (ENO1) controls alpha v/beta 3 integrin expression and regulates pancreatic cancer adhesion, invasion, and metastasis. J Hematol Oncol 10:16
Hirschhaeuser F, Sattler UG, Mueller-Klieser W (2011) Lactate: a metabolic key player in cancer. Cancer Res 71:6921–6925
Nikitovic D, Kouvidi K, Karamanos NK, Tzanakakis GN (2013) The roles of hyaluronan/RHAMM/CD44 and their respective interactions along the insidious pathways of fibrosarcoma progression. Biomed Res Int 2013:929531
Torres MP et al (2012) Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett 323:29–40
Xu W-Y et al (2018) Zinc finger E-box-binding homeobox 1 mediates aerobic glycolysis via suppression of sirtuin 3 in pancreatic cancer. World J Gastroenterol 24:4893–4905
Funasaka T, Raz A (2007) The role of autocrine motility factor in tumor and tumor microenvironment. Cancer Metastasis Rev 26:725–735
Amin S, Yang P, Li Z (2019) Pyruvate kinase M2: a multifarious enzyme in non-canonical localization to promote cancer progression. BBA-Rev Cancer 1871:331–341
Ristic B, Bhutia YD, Ganapathy V (2017) Cell-surface G-protein-coupled receptors for tumor-associated metabolites: a direct link to mitochondrial dysfunction in cancer. Biochim Biophys Acta Rev Cancer 1868:246–257
Wagner W, Ciszewski WM, Kania KD (2015) l- and d-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun Signal 13:36
Colegio OR et al (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513:559–563
Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O (2011) Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res 71:2550–2560
Xu Y et al (2014) Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol 34:1231–1239
Ziegler ME, Hatch MM, Wu N, Muawad SA, Hughes CC (2016) mTORC2 mediates CXCL12-induced angiogenesis. Angiogenesis 19:359–371
Redman RA, Pohlmann PR, Kurman MR, Tapolsky G, Chesney JA (2015) A phase I, dose-escalation, multi-center study of PFK-158 in patients with advanced solid malignancies explores a first-in-man inhibitor of glycolysis. J Clin Oncol 33:TPS2606. https://doi.org/10.1200/jco.2015.33.15_suppl.tps2606
Conradi LC et al (2017) Tumor vessel disintegration by maximum tolerable PFKFB3 blockade. Angiogenesis 20:599–613
De Bock K et al (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154:651–663
Labelle M, Hynes RO (2012) The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov 2:1091
Labelle M, Begum S, Hynes Richard O (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20:576–590
Coffelt SB et al (2015) IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522:345. https://doi.org/10.1038/nature14282
Spiegel A et al (2016) Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov 6:630
Peiris-Pages M, Martinez-Outschoorn UE, Sotgia F, Lisanti MP (2015) Metastasis and oxidative stress: are antioxidants a metabolic driver of progression? Cell Metab 22:956–958
Massague J, Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529:298–306
Cheng TY et al (2018) Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene 37:1730–1742
Penny HL et al (2016) Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology 5:1191731
Husain Z, Huang Y, Seth P, Sukhatme VP (2013) Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol 191:1486
Patel S et al (2018) Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol 19:1236–1247
Yachida S, Iacobuzio-Donahue CA (2009) The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med 133:413–422
Fabian A et al (2019) Metastasis of pancreatic cancer: An uninflamed liver micromilieu controls cell growth and cancer stem cell properties by oxidative phosphorylation in pancreatic ductal epithelial cells. Cancer Lett 453:95–106
Brandi J et al (2017) Proteomic analysis of pancreatic cancer stem cells: functional role of fatty acid synthesis and mevalonate pathways. J Proteomics 150:310–322
Viale A et al (2014) Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514:628–632
Zhou C et al (2018) Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth. Cell Death Dis 9:161. https://doi.org/10.1038/s41419-017-0196-z
Rademaker G et al (2019) Myoferlin contributes to the metastatic phenotype of pancreatic cancer cells by enhancing their migratory capacity through the control of oxidative phosphorylation. Cancers 11:853
LeBleu VS et al (2014) PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16:992–1003
Redman RA, Pohlmann PR, Kurman MR, Tapolsky G, Chesney J (2015) A phase I, dose-escalation, multicenter study of ACT-PFK-158, 2HCl in patients with advanced solid malignancies explores a first-in-human inhibitor of glycolysis. J Clin Oncol 33. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L71836038. Accessed 17 Aug 2019
Telang S et al (2016) PFK-158 is a first-in-human inhibitor of PFKFB3 that selectively suppresses glucose metabolism of cancer cells and inhibits the immunosuppressive Th17 cells and MDSCs in advanced cancer patients. Cancer Res 76. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L617902344. Accessed 17 Aug 2019
Chapiro J et al (2014) Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer. Clin Cancer Res 20:6406–6417
Patra KC et al (2013) Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24:213–228
Maftouh M et al (2014) Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer 110:172–182
Le A et al (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. P Natl Acad Sci USA 107:2037. http://www.pnas.org/content/107/5/2037.abstract. Accessed 17 Aug 2019
Hui S et al (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551:115–118
Deng S-J et al (2019) Nutrient stress–dysregulated antisense lncRNA GLS-AS impairs GLS-mediated metabolism and represses pancreatic cancer progression. Cancer Res 79:1398. http://cancerres.aacrjournals.org/content/79/7/1398.abstract. Accessed 17 Aug 2019
Son J et al (2013) Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496:101–105
Li W et al (2017) HIF-2α regulates non-canonical glutamine metabolism via activation of PI3 K/mTORC2 pathway in human pancreatic ductal adenocarcinoma. J Cell Mol Med 21:2896–2908. https://doi.org/10.1111/jcmm.13202
Swierczynski J, Hebanowska A, Sledzinski T (2014) Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J Gastroenterol 20:2279–2303
Guillaumond F et al (2015) Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. P Natl Acad Sci USA 112:2473–2478
Boudreau A et al (2016) Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat Chem Biol 12:779–786
Draoui N, de Zeeuw P, Carmeliet P (2017) Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biol 7:170219
Makohon-Moore AP et al (2017) Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet 49:358–366
Qin Y, Cheng C, Lu H, Wang Y (2016) miR-4458 suppresses glycolysis and lactate production by directly targeting hexokinase2 in colon cancer cells. Biochem Biophys Res Commun 469:37–43
Lin YH et al (2018) Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology 67:188–203
Rong Y et al (2013) Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol 34:1523–1530
Yang B et al (2017) Overexpression of lncRNA IGFBP4-1 reprograms energy metabolism to promote lung cancer progression. Mol Cancer 16:154
Bhattacharya R, Ray Chaudhuri S, Roy SS (2018) FGF9-induced ovarian cancer cell invasion involves VEGF-A/VEGFR2 augmentation by virtue of ETS1 upregulation and metabolic reprogramming. J Cell Biochem 119:8174–8189
Liotta LA et al (1986) Tumor cell autocrine motility factor. Proc Natl Acad Sci USA 83:3302. http://www.pnas.org/content/83/10/3302.abstract. Accessed 17 Aug 2019
Timar J et al (1996) Autocrine motility factor signals integrin-mediated metastatic melanoma cell adhesion and invasion. Cancer Res 56:1902–1908
Li Y, Wei Z, Dong B, Lian Z, Xu Y (2016) Silencing of phosphoglucose isomerase/autocrine motility factor decreases U87 human glioblastoma cell migration. Int J Mol Med 37:998–1004
Gallardo-Perez JC, Adan-Ladron de Guevara A, Marin-Hernandez A, Moreno-Sanchez R, Rodriguez-Enriquez S (2017) HPI/AMF inhibition halts the development of the aggressive phenotype of breast cancer stem cells. BBA-Mol Cell Res 1864:1679–1690
Funasaka T, Yanagawa T, Hogan V, Raz A (2005) Regulation of phosphoglucose isomerase/autocrine motility factor expression by hypoxia. Faseb J 19:1422–1430
Mishra S, Raz A, Murphy LJ (2004) Insulin-like growth factor binding protein-3 interacts with autocrine motility factor/phosphoglucose isomerase (AMF/PGI) and inhibits the AMF/PGI function. Cancer Res 64:2516. http://cancerres.aacrjournals.org/content/64/7/2516.abstract
Ahmad A et al (2011) Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res 71:3400–3409
Haga A, Funasaka T, Deyashiki Y, Raz A (2008) Autocrine motility factor stimulates the invasiveness of malignant cells as well as up-regulation of matrix metalloproteinase-3 expression via a MAPK pathway. FEBS Lett 582:1877–1882
Gu M et al (2017) PFKFB3 promotes proliferation, migration and angiogenesis in nasopharyngeal carcinoma. J Cancer 8:3887–3896
Han J, Meng Q, Xi Q, Wang H, Wu G (2017) PFKFB3 was overexpressed in gastric cancer patients and promoted the proliferation and migration of gastric cancer cells. Cancer Biomark 18:249–256
Peng F et al (2018) PFKFB3 is involved in breast cancer proliferation, migration, invasion and angiogenesis. Int J Oncol 52:945–954
Ros S et al (2012) Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov 2:328–343
Yun SJ et al (2012) PFKFB4 as a prognostic marker in non-muscle-invasive bladder cancer. Urol Oncol 30:893–899
Dasgupta S et al (2018) Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer. Nature 556:249–254
Cheung Eric C et al (2013) TIGAR is required for efficient intestinal regeneration and tumorigenesis. Dev Cell 25:463–477. https://doi.org/10.1016/j.devcel.2013.05.001
Wanka C, Steinbach JP, Rieger J (2012) Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. J Biol Chem 287:33436–33446
Won KY et al (2012) Regulatory role of p53 in cancer metabolism via SCO2 and TIGAR in human breast cancer. Hum Pathol 43:221–228
Qian S et al (2016) TIGAR cooperated with glycolysis to inhibit the apoptosis of leukemia cells and associated with poor prognosis in patients with cytogenetically normal acute myeloid leukemia. J Hematol Oncol 9:128
Liu J et al (2018) High expression of synthesis of cytochrome c oxidase 2 and TP53-induced glycolysis and apoptosis regulator can predict poor prognosis in human lung adenocarcinoma. Hum Pathol 77:54–62
Kim SH, Choi SI, Won KY, Lim SJ (2016) Distinctive interrelation of p53 with SCO2, COX, and TIGAR in human gastric cancer. Pathol Res Pract 212:904–910
Shen M et al (2018) Met is involved in TIGAR-regulated metastasis of non-small-cell lung cancer. Mol Cancer 17:88
Funding
This study was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018PT32014), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2 M-1-001), and the National Nature Science Foundation of China (81672960; 81672443).
Author information
Authors and Affiliations
Contributions
Study concept and design: All authors. Drafting of the manuscript: JY and BR. Critical revision of the manuscript for important intellectual content: GY, HW and GC. Obtained the funding: LY, TZ and YZ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Ren, B., Yang, G. et al. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell. Mol. Life Sci. 77, 305–321 (2020). https://doi.org/10.1007/s00018-019-03278-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03278-z