Abstract
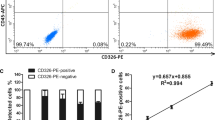
To establish a novel molecular diagnostic method of detecting circulating tumor cells (CTCs) LS174T colon cancer cells were serially diluted with normal blood. Additional peripheral blood samples were collected from 25 patients with colorectal carcinoma. Mononuclear cells (MNCs) were collected, equally divided into four parts, and then cancer cells were enriched by four methods: method A, nonimmunobead method; method B, negative immunobead method: CD45 immunomagnetic beads were used to deplete the leukocytes; method C, positive immunobead method: Ber-EP4 immunomagnetic beads were used to enrich cancer cells; method D, negative-and-positive immunobead method: CD45 immunomagnetic beads were first used to deplete the leukocytes from MNC and then Ber-EP4 immunomagnetic beads were used to enrich cancer cells. Finally, real-time quantitative RT-PCR was used to monitor mRNA expression of β2-mircoglobulin (β2M) and carcinoembryonic antigen (CEA). The relative CEA mRNA values were corrected with reference to β2M mRNA, to CEA mRNA/β2M mRNA ratios according to a CEA mRNA external standards prepared with tenfold serial dilutions (1–104 IS174T cells) of cDNA and β2M mRNA external standards prepared with tenfold serial dilutions (102–107 leukocytes) of cDNA. In recovery experiments a significant correlation between the number of cancer cells and CEA mRNA expression was found when CD45 or Ber-EP4 immunomagnetic beads were used alone. A highly significant correlation was found when CD45 and Ber-EP4 immunomagnetic beads were used successively. The sensitivity of method D was one cancer cell per milliliter of blood. Circulating cancer cells were detected in 19 of 25 patients with colorectal cancers. The relative CEA mRNA value obtained by method D was the smallest. The positive detection rate of circulating cancer cells in patients at Dukes’ B, C, and D stages were 25.0% (1/4), 83.3% (10/12), and 88.9% (8/9). Combinative use of immunomagnetic isolation followed by real-time RT-PCR is a useful technique to detect circulating tumor cells in patients with colorectal carcinomas. Applying negative and positive immunomagnetic beads successively yields the highest correlation with amount of tumor cells.




Similar content being viewed by others
Abbreviations
- CEA :
-
Carcinoembryonic antigen
- Cp :
-
Crossing point
- CTC :
-
Circulating tumor cell
- MNC :
-
Mononuclear cell
- PBL :
-
Peripheral blood leukocyte
- PCR :
-
Polymerase chain reaction
- RT :
-
Reverse transcription
- β2M :
-
β2-Mircoglobulin
References
White H, Griffiths JD (1976) Circulating malignant cells and fibrinolysis during resection of colorectal cancer. Proc R Soc Med 69:467
Ghossein RA, Rosai J (1996) Polymerase chain reaction in the detection of micrometastases and circulating tumor cells. Cancer 78:10
Pelkey TJ, Frierson HF Jr, Bruns DE (1996) Molecular and immunological detection of circulating tumor cells and micrometastases from solid tumors. Clin Chem 42:1369
Pantel K, Cote RJ, Fodstad O (1999) Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst 91:1113
Lambrechts AC, van ‘t Veer LJ, Rodenhuis S (1998) The detection of minimal numbers of contaminating epithelial tumor cells in blood or bone marrow: use, limitations and future of RNA-based methods. Ann Oncol 9:1269
Vlems FA, Diepstra JH, Cornelissen IM, Ruers TJ, Ligtenberg MJ, Punt CJ, van Krieken JH, Wobbes T, van Muijen GN (2002) Limitations of cytokeratin 20 RT-PCR to detect disseminated tumor cells in blood and bone marrow of patients with colorectal cancer: expression in controls and downregulation in tumour tissue. Mol Pathol 55:156
Hardingham JE, Kotasek D, Farmer B, Butler RN, Mi JX, Sage RE, Dobrovic A (1993) Immunobead-PCR: a technique for the detection of circulating tumor cells using immunomagnetic beads and the polymerase chain reaction. Cancer Res 53:3455
Park S, Lee B, Kim I, Choi I, Hong K, Ryu Y, Rhim J, Shin J, Park SC, Chung H, Chung J (2001) Immunobead RT-PCR versus regular RT-PCR amplification of CEA mRNA in peripheral blood. J Cancer Res Clin Oncol 127:489
Cremoux P de, Extra JM, Denis MG, Pierga JY, Bourstyn E, Nos C, Clough KB, Boudou E, Martin EC, Muller A, Pouillart P, Magdelenat H (2000) Detection of MUC1-expressing mammary carcinoma cells in the peripheral blood of breast cancer patients by real-time polymerase chain reaction. Clin Cancer Res 6:3117
Sheibani K, Shin SS, Kezirian J, Weiss LM (1991) Ber-EP4 antibody as a discriminant in the differential diagnosis of malignant mesothelioma versus adenocarcinoma. Am J Surg Pathol 15:779
Latza U, Niedobitek G, Schwarting R, Nekarda H, Stein H (1990) Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelial. J Clin Pathol 43:213
Iinuma H, Okinaga K, Adachi M, Suda K, Sekine T, Sakagawa K, Baba Y, Tamura J, Kumagai H, Ida A (2000) Detection of tumor cells in blood using CD45 magnetic cell separation followed by nested mutant allele-specific amplification of p53 and K-ras genes in patients with colorectal cancer. Int J Cancer 89:337
Ito S, Nakanishi H, Hirai T, Kato T, Kodera Y, Feng Z, Kasai Y, Ito K, Akiyama S, Nakao A, Tatematsu M (2002) Quantitative detection of CEA expressing free tumor cells in the peripheral blood of colorectal cancer patients during surgery with real-time RT-PCR on a LightCycler. Cancer Lett 183:195
Burchill SA, Bradbury MF, Pittman K, Southgate J, Smith B, Selby P (1995) Detection of epithelial cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. Br J Cancer 71:278
Gerhard M, Juhl H, Kalthoff H, Schreiber HW, Wagener C, Neumaier M (1994) Specific detection of carcinoembryonic antigen-expressing tumor cells in bone marrow aspirates by polymerase chain reaction. J Clin Oncol 12:725
Emig M, Saussele S, Wittor H, Weisser A, Reiter A, Willer A, Berger U, Hehlmann R, Cross NC, Hochhaus A (1999) Accurate and rapid analysis of residual disease in patients with CML using specific fluorescent hybridization probes for real time quantitative RT-PCR. Leukemia 13:1825
Bostick PJ, Chatterjee S, Chi DD, Huynh KT, Giuliano AE, Cote R, Hoon DS (1998) Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol 16:2632
Hardingham JE, Hewett PJ, Sage RE, Finch JL, Nuttall JD, Kotasek D, Dobrovic A (2000) Molecular detection of blood-borne epithelial cells in colorectal cancer patients and in patients with benign bowel disease. Int J Cancer 89:8
Raynor M, Stephenson SA, Walsh DC, Pittman KB, Dobrovic A (2002) Optimisation of the RT-PCR detection of immunomagnetically enriched carcinoma cells. BMC Cancer 2:14
Degan M, Mazzocco FT, Di Francia R, Rossi FM, Pinto A, Gattei V (2000) Normalizing complementary DNA by quantitative reverse transcriptase-polymerase chain reaction of beta2-microglobulin: molecular monitoring of minimal residual disease in acute promyelocytic leukemia. Diagn Mol Pathol 9:98
Acknowledgements
This work was supported by Key Doctoral Fund of Ningbo Municipality (02J20101-07), Medical Sciences Research Fund of Zhejiang Province (2002A073), and Key Research Project of Ningbo University (z0214015). We thank Dr. Dieter Soll, Yale University, USA, for his kind help in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, J., Xiao, B., Zhang, X. et al. Combined use of positive and negative immunomagnetic isolation followed by real-time RT-PCR for detection of the circulating tumor cells in patients with colorectal cancers. J Mol Med 82, 768–774 (2004). https://doi.org/10.1007/s00109-004-0590-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-004-0590-8