Abstract
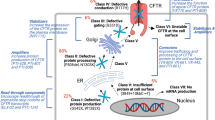
Cystic fibrosis (CF) is the most common lethal inherited disease in Caucasians and is caused by mutations in the CFTR gene. The disease is incurable and medical treatment is limited to the amelioration of symptoms or secondary complications. A comprehensive understanding of the disease mechanisms and the development of novel treatment options require appropriate animal models. Existing CF mouse models fail to reflect important aspects of human CF. We thus generated a CF pig model by inactivating the CFTR gene in primary porcine cells by sequential targeting using modified bacterial artificial chromosome vectors. These cells were then used to generate homozygous CFTR mutant piglets by somatic cell nuclear transfer. The homozygous CFTR mutants lack CFTR protein expression and display severe malformations in the intestine, respiratory tract, pancreas, liver, gallbladder, and male reproductive tract. These phenotypic abnormalities closely resemble both the human CF pathology as well as alterations observed in a recently published CF pig model which was generated by a different gene targeting strategy. Our new CF pig model underlines the value of the CFTR-deficient pig for gaining new insight into the disease mechanisms of CF and for the development and evaluation of new therapeutic strategies. This model will furthermore increase the availability of CF pigs to the scientific community.






Similar content being viewed by others
References
Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN et al (2011) Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros 10(Suppl 2):S152–S171
Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT et al (2008) The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 295:L240–L263
Grubb BR, Boucher RC (1999) Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev 79:S193–S214
Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A et al (1994) Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(−/−) mice. Proc Natl Acad Sci USA 91:479–483
Gray MA, Winpenny JP, Porteous DJ, Dorin JR, Argent BE (1994) CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic CF mouse. Am J Physiol 266:C213–C221
Norkina O, De Lisle RC (2005) Potential genetic modifiers of the cystic fibrosis intestinal inflammatory phenotype on mouse chromosomes 1, 9, and 10. BMC Genet 6:29
Zhou Z, Duerr J, Johannesson B, Schubert SC, Treis D et al (2011) The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros 10(Suppl 2):S172–S182
Sun X, Yan Z, Yi Y, Li Z, Lei D et al (2008) Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest 118:1578–1583
Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA et al (2008) Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest 118:1571–1577
Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T et al (2008) Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321:1837–1841
Sun X, Sui H, Fisher JT, Yan Z, Liu X et al (2010) Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 120:3149–3160
Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA et al (2010) Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med 182:1251–1261
Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP et al (2010) Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2:29ra31
Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ (2010) Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol 176:1377–1389
Pierucci-Alves F, Akoyev V, Stewart JC 3rd, Wang LH, Janardhan KS et al (2011) Swine models of cystic fibrosis reveal male reproductive tract phenotype at birth. Biol Reprod 85:442–451
Chang EH, Lacruz RS, Bromage TG, Bringas P Jr, Welsh MJ et al (2011) Enamel pathology resulting from loss of function in the cystic fibrosis transmembrane conductance regulator in a porcine animal model. Cells Tissues Organs 194:249–254
Itani OA, Chen JH, Karp PH, Ernst S, Keshavjee S et al (2011) Human cystic fibrosis airway epithelia have reduced Cl− conductance but not increased Na+ conductance. Proc Natl Acad Sci USA 108:10260–10265
Rogan MP, Reznikov LR, Pezzulo AA, Gansemer ND, Samuel M et al (2010) Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci USA 107:20571–20575
Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC (1991) Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153–1163
Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG (2005) Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33:e36
Sauer B (1993) Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Methods Enzymol 225:890–900
Kurome M, Ueda H, Tomii R, Naruse K, Nagashima H (2006) Production of transgenic-clone pigs by the combination of ICSI-mediated gene transfer with somatic cell nuclear transfer. Transgenic Res 15:229–240
Besenfelder U, Modl J, Muller M, Brem G (1997) Endoscopic embryo collection and embryo transfer into the oviduct and the uterus of pigs. Theriogenology 47:1051–1060
Plog S, Mundhenk L, Bothe MK, Klymiuk N, Gruber AD (2010) Tissue and cellular expression patterns of porcine CFTR: similarities to and differences from human CFTR. J Histochem Cytochem 58:785–797
Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL et al (2002) Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295:1089–1092
Jin DI, Lee SH, Choi JH, Lee JS, Lee JE et al (2003) Targeting efficiency of a-1,3-galactosyl transferase gene in pig fetal fibroblast cells. Exp Mol Med 35:572–577
Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD et al (2003) Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 299:411–414
Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN et al (2003) High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol 21:652–659
Vajta G, Zhang Y, Machaty Z (2007) Somatic cell nuclear transfer in pigs: recent achievements and future possibilities. Reprod Fertil Dev 19:403–423
Guilbault C, Saeed Z, Downey GP, Radzioch D (2007) Cystic fibrosis mouse models. Am J Respir Cell Mol Biol 36:1–7
Salmon H, Berri M, Gerdts V, Meurens F (2009) Humoral and cellular factors of maternal immunity in swine. Dev Comp Immunol 33:384–393
Ostedgaard LS, Meyerholz DK, Vermeer DW, Karp PH, Schneider L et al (2011) Cystic fibrosis transmembrane conductance regulator with a shortened R domain rescues the intestinal phenotype of CFTR−/− mice. Proc Natl Acad Sci USA 108:2921–2926
Acknowledgments
This study was supported by the Mukoviszidose Institut gemeinnützige Gesellschaft für Forschung und Therapieentwicklung mbH and - in part - by the Bundesministerium für Bildung und Forschung (Leading Edge Cluster Munich: m4, Personalized Medicine and Targeted Therapies). We thank Tuna Güngör, Christian Erdle, Siegfried Elsner, Jana Enders, Gabriele Hahn, Alexandra Harder, and Petra Nehrig for excellent technical support, and Lynda Ostedgaard and Michael J. Welsh for donation of the pCFTR clone.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Klymiuk, N., Mundhenk, L., Kraehe, K. et al. Sequential targeting of CFTR by BAC vectors generates a novel pig model of cystic fibrosis. J Mol Med 90, 597–608 (2012). https://doi.org/10.1007/s00109-011-0839-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0839-y