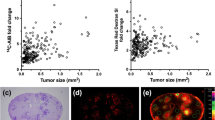
Abstract
Breast cancer that has metastasized to the brain presents difficult clinical challenges. This diagnosis comes with high mortality rates, largely due to complexities in early detection and ineffective therapies associated with both dormancy and impermeability of the blood–brain barrier (BBB). Magnetic resonance imaging (MRI) is the current gold standard for diagnosis and assessment of brain tumors. It has been used clinically to investigate metastatic development as well as monitor response to therapy. Here, we describe preclinical imaging strategies that we have used to study the development of brain metastases due to breast cancer. Using this approach, we have identified three subsets of metastatic disease: permeable metastases, nonpermeable metastases, and solitary, dormant cancer cells, which likely have very different biology and responses to therapy. The ability to simultaneously monitor the spatial and temporal distribution of dormant cancer cells, metastatic growth, and associated tumor permeability can provide great insight into factors that contribute to malignant proliferation. Our preclinical findings suggest that standard clinical detection strategies may underestimate the true metastatic burden of breast cancer that has metastasized to the brain. A better understanding of true metastatic burden in brains will be important to assist in the development of more effective chemotherapeutics—particularly those targeted to cross the BBB—as well as detection of small nonpermeable metastases.




Similar content being viewed by others
References
Breast Cancer Facts 2013. http://www.nationalbreastcancer.org/breast-cancer-facts. Accessed 16 Oct 2013
Canadian Cancer Statistics 2013. http://www.cancer.ca. Accessed 3 Sept 2013
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572
Maxmen A (2012) The hard facts. Nature 485:S50–S51
Weiss L (1990) Metastatic inefficiency. Adv Cancer Res 54:159–211
Tarin D, Vass AC, Kettlewell MG, Price JE (1984) Absence of metastatic sequelae during long-term treatment of malignant ascites by peritoneo-venous shunting. A clinic-pathological report. Invas Metast 4:1–12
Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B (1984) Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res 44:3584–3592
Goss PE, Chambers AF (2010) Does tumour dormancy offer a therapeutic target? Nat Rev Cancer 10(12):871–877
Canadian Cancer Statistics 2010. http://www.cancer.ca Accessed Feb 2010
Sharma M, Abraham J (2007) CNS metastasis in primary breast cancer. Expert Rev Anticancer Ther 7(11):1561–1566
Kirsch DG, Loeffler JS (2005) Brain metastases in patients with breast cancer: new horizons. Clin Breast Cancer 2:115–124
Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J et al (2006) Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 17(6):935–944
Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP (2008) Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113(10):2638–2645
Sperduto PW, Kased N, Roberge D, XU Z, Shanley R, Luo X, Sneed P, Chao ST et al (2012) Effect of tumor subtype on survival and the graded prognostic assessment for patients breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 82(5):2111–2117
Vern-Gross TZ, Lawrence JA, Case LD, McMullen KP, Bourland JD, Metheny-Barlow LJ, Ellis TL, Shaw EG et al (2012) Breast cancer subtype affects patterns of failure of brain metastases after treatment with stereotactic radiosurgery. J Neurooncol 110(3):381–388
MacDonald IC, Chambers AF (2006) Breast cancer metastasis progression as revealed by intravital videomicroscopy. Expert Rev Anticancer Ther 6:1271–1279
Allan AL, Vantyghem SA, Tuck AB, Chambers AF (2006-2007) Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis 26:87-89
Barkan D, Chambers AF (2011) β1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clin Cancer Res 17(23):7219–7223
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY et al (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15(7):807–817
Sosa MS, Bragado P, Debnath J, Aguirre-Ghiso JA (2013) Regulation of tumor cell dormancy by tissue microenvironments and autophagy. Adv Exp Med Biol 734:73–89
Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VHC, Groom AC, Chambers AF (2003) Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat 82(3):199–206
Townson JT, Ramadan SS, Simedrea C, Rutt BK, MacDonald IC, Foster PJ, Chambers AF (2009) Three-dimensional imaging and quantification of both solitary cells and metastases in whole mouse liver by magnetic resonance imaging. Cancer Res 69(21):8326–8331
Ohno S, Ishida M, Kataoka A, Murakami S (2004) Brain metastasis of breast cancer. Breast Cancer 11(1):27–29
Mokbel K, Hassanally D (2001) From HER2 to herceptin. Curr Med Res Opin 17(1):51–59
Baselga J, Albanell J (2001) Mechanism of action of anti-HER2 monoclonal antibodies. Ann Oncol 12(Suppl1):S35–S41
Daniele L, Sapino A (2009) Anti-HER2 treatment and breast cancer: state of the art, recent patents and new strategies. Recent Pat Anticancer Drug Discov 4(1):9–18 (10)
Gonzalez-Angulo AM, Hortobagyi GN, Esteva FJ (2006) Adjuvant therapy with trastuzumab for HER2/neu-positive breast cancer. Oncologist 11(8):857–867
Pieńkowski T, Zielinski CC (2010) Trastuzumab treatment in patients with breast cancer and metastatic CNS disease. Ann Oncol 21(5):917–924
Shmueli E, Wigler N, Inbar M (2004) Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer 40(3):379–382
Clayton A, Danson S, Jolly S, Ryder W, Burt P, Stewart A et al (2004) Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 91:639–643
Lower E, Blau R, Bismayer J, Brennan L, Broun R, Dannenman W et al (2001) Increased brain metastasis detected in metastatic breast cancer patients receiving herceptin. Breast Cancer Res Treat 69:271
Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA (2012) Outcomes after whole brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys 82(2):e167–e172
Anzalone N, Gerevini S, Scotti R, Vezzulli P, Picozzi P (2009) Detection of cerebral metastases on magnetic resonance imaging: intraindividual comparison of gadobutrol with gadopentetate dimeglumine. Acta Radiol 50(8):933–940
Runge VM, Ai T, Hao D, Hu X (2011) The developmental history of the use of gadolinium chelates as intravenous contrast media for MRI. Invest Radiol 46(12):807
van der Molen AJ, Bellin MF (2008) Extracellular gadolinium-based contrast media. Eur J Radiol 66(2):168–174
Padhani AR (2002) Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging 16(4):407–422
Schaefer PW, Grant PE, Gonzalez RG (2000) Diffusion-weighted MR imaging of the brain. Radiology 217:331–345
Rye PD, Norum L, Olsen DR et al (1996) Brain metastasis model in athymic nude mice using a novel MUC1-secreting human breast-cancer cell line, MA11. Int J Cancer 68(5):682–687
Yoneda T, Williams PJ, Hiraga T et al (2001) A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res 16(8):1486–1495
Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, Kurek R, Vega-Valle E, Feigenbaum L, Halverson D et al (2007) Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res 67(9):4190–4198
Tanner M, Kapanen AI, Raheem O, Grenman S, Elo J, Elenius K, Isola J (2004) Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther 3(12):1585–1592
Quintana A, Raczka E, Bonaccorsi A (1979) Cardiac output distribution measured with radioactive microspheres in the mouse. Pharmacol Res Commun 11(3):245–252
Basse P, Hokland P, Heron I, Hokland M (1988) Fate of tumour cells injected into left ventricle of heart in BALB/c mice: role of natural killer cells. J Natl Cancer Inst 80(9):657–665
Perera M, Ribot EJ, Percy DB, McFadden C, Simedrea C, Palmieri D, Chambers AF, Foster PJ (2012) In vivo magnetic resonance imaging for investigating the development and distribution of experimental brain metastases due to breast cancer. Transl Oncol 5(3):1–9
Heyn C, Ronald JA, Mackenzie LT, MacDonald IC, Chambers AF, Rutt BK, Foster PJ (2006) In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med 55:23–29
Gonzales-Lara LE, Xu X, Hofstetrova K, Pniak A, Chen Y, McFadden C, Martinez-Santiesteban FM, Rutt BK, Brown A, Foster PJ (2011) The use of cellular magnetic resonance imaging to track the fate of iron-labelled multipotent stromal cells after direct transplantation in a mouse model of spinal cord injury. Mol Imaging Biol 13(4):702–711
Noad J, Gonzales-Lara LE, Broughton HC, McFadden C, Chen Y, Hess DA, Foster PJ (2013) MRI tracking of transplanted iron-labeled mesenchymal stromal cells in an immune-compromised mouse model of critical limb ischemia. NMR Biomed 26(4):458–467
Lu S, Liu S, Zu Q, Xu X, Yu J, Wang J, Zhang Y, Shi H (2013) In vivo MR imaging of intraarterially delivered magnetically labeled mesenchymal stem cells in a canine stroke model. PLoS ONE 8(2):e54963
Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM, MacKenzie LT, Mikulis DJ, Palmieri D, Bronder JL, Steeg PS et al (2006) In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med 56:1001–1010
Zhang RD, Price JE, Fujimaki T et al (1992) Differential permeability of the blood–brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol 141:1115–1124
Lockman PR, Mittapalli RK, Taskar KS et al (2010) Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 16:5664–5678
Percy DB, Ribot EJ, Chen Y, McFadden C, Simedrea C, Steeg PS, Chambers AF, Foster PJ (2011) In vivo characterization of changing blood–tumor barrier permeability in a mouse model of breast cancer metastasis: a complementary magnetic resonance imaging approach. Invest Radiol 46(11):718–725
Hermanek P, Hutter RV, Sobin LH, Wittekind C (1999) Classification of isolated tumor cells and micrometastasis. Cancer 86:2668–2673
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Marksbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med 322:494–500
Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B (1998) Postoperative radiotherapy in the treatment of single metastases to the brain—A randomized trial. J Amer Med Assoc 280(17):1485–1489
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase ΙΙΙ results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Huang F, Alrefae M, Langleben A, Roberge D (2009) Prophylactic cranial irradiation in advanced breast cancer: a case for caution. Int J Radiat Oncol 73(3):752–758
Acknowledgments
We thank Chelsey Gareau for preparation of Fig. 4. Work described in this review is supported by a grant from the US Department of Defense Breast Cancer Research Program (#W81XWH-06–2-0033). DBP is supported by studentships from the Canadian Institutes of Health Research Strategic Training Program in Cancer Research and Technology and the Translational Breast Cancer Research Unit of the London Regional Cancer Program. AFC is Canada Research Chair in Oncology and receives salary support from the Canada Research Chairs Program.
Disclosure statement
The authors have no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murrell, D.H., Foster, P.J. & Chambers, A.F. Brain metastases from breast cancer: lessons from experimental magnetic resonance imaging studies and clinical implications. J Mol Med 92, 5–12 (2014). https://doi.org/10.1007/s00109-013-1108-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-013-1108-z