Abstract
Background
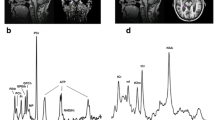
Abnormalities of membrane phospholipid metabolism have been described in Alzheimer’s disease (AD). We investigated, with the aid of 31P magnetic resonance spectroscopy, the in vivo intracerebral availability of phosphomonoesters (PME) and phosphodiesters (PDE) in patients with AD.
Methods
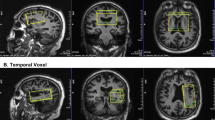
Eighteen outpatients with mild or moderate probable AD and 16 nondemented elderly volunteers were assessed with the Cambridge Examination for Mental Disorders of the Elderly (CAMDEX) and its cognitive subscale of the CAMDEX schedule (CAMCOG). Scans were performed on a 1.5 T magnetic resonance imager addressing a 40-cm3 voxel in the left prefrontal cortex. Main outcome measures were mean relative peak areas of PME and PDE, which provide an estimate of membrane phospholipid metabolism.
Results
PME resonance and the PME/PDE ratio were increased in AD patients as compared to controls (p<0.05). PME was negatively correlated with global cognitive performance as shown by the Mini-Mental State Examination (rs=−0.36, p=0.05) and CAMCOG scores (rs=−0.49, p=0.007), as well as with discrete neuropsychological functions, namely, memory (rs=−0.53, p=0.004), visual perception (rs=−0.54, p=0.003), orientation (rs=−0.36, p=0.05), and abstract thinking (rs=−0.48, p=0.01).
Conclusions
We provide evidence of reduced membrane phospholipid breakdown in the prefrontal cortex of mild and moderately demented AD patients. These abnormalities correlate with neuropsychological deficits that are characteristic of AD.



Similar content being viewed by others
References
Blessed G, Tomlinson BE, Roth M (1969) The association between quantitative measures and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 114:797–811
Blusztajn JK, Liscovitch M, Richardson UI (1987) Synthesis of acethylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci U S A 84(15):5474–5477
Bottino CMC, Almeida OP, Tamai S, Forlenza OV, Scalco MZ, Carvalho IAM (1999) Entrevista estruturada para diagnóstico de transtornos mentais em idosos—CAMDEX The Cambridge Examination for Mental Disorders of the Elderly. Cambridge University Press (Brazilian version, translated and adapted on behalf of the editors)
Bottomley PA, Cousins JP, Pendrey DL, Wagle WA, Hardy CJ, Eames FA et al (1992) Alzheimer dementia: quantification of energy metabolism and mobile phosphoesters with P-31 NMR spectroscopy. Radiology 183:695–699
Brown GG, Levine SR, Gorell JM, Pettegrew JW, Gdowski JW, Bueri JA et al (1989) In vivo 31P NMR profiles of Alzheimer’s disease and multiple subcortical infarct dementia. Neurology 39(11):1423–1427
Brown GG, Garcia JH, Gdowski JW, Levine SR, Helpern JÁ (1993) Altered brain energy metabolism in demented patients with multiple subcortical ischemic lesions. Working hypotheses. Arch Neurol 50(4):384–8
Cuenod CA, Kaplan DB, Michot JL, Jehenson P, Leroy-Willig A, Forette F et al (1995) Phospholipid abnormalities in early Alzheimer’s disease. In vivo phosphorus 31 magnetic resonance spectroscopy. Arch Neurol 52(1):89–94
Dennis EA (1994) Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem 269:13057–13060
Eckert GP, Cairns NJ, Maras A, Gattaz WF, Muller WE (2000) Cholesterol modulates the membrane-disordering effects of beta-amyloid peptides in the hippocampus: specific changes in Alzheimer’s disease. Dement Geriatr Cogn Disord 11(4):181–186
Emmerling MR, Moore CJ, Doyle PD, Carroll RT, Davis RE (1993) Phospholipase A2 activation influences the processing and secretion of the amyloid precursor protein. Biochem Biophys Res Commun 197(1):292–297
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Gattaz WF, Köllisch M, Thuren T, Virtanen JA, Kinnunen PKJ (1987) Increased plasma phospholipase A2 activity in schizophrenic patients: reduction after neuroleptic therapy. Biol Psychiatry 22:421–426
Gattaz WF, Maras A, Cairns NJ, Levy R, Förstl H (1995a) Decreased phospholipase A2 activity in Alzheimer brains. Biol Psychiatry 37:13–17
Gattaz WF, Steudle A, Maras A (1995b) Increased platelet phospholipase A2 in schizophrenia. Schizophr Res 16:1–6
Gattaz WF, Cairns NJ, Levy R, Forstl H, Braus DF, Maras A (1996) Decreased phospholipase A2 activity in the brain and in platelets of patients with Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 246(3):129–131
Gattaz WF, Forlenza OV, Talib LL, Barbosa NR, Bottino CM (2004) Platelet phospholipase A2 activity in Alzheimer’s disease and mild cognitive impairment. J Neural Transm 111(5):591–601
Gonzalez RG, Guimaraes AR, Moore GJ, Crawley A, Cupples LA, Growdon JH (1996) Quantitative in vivo 31P magnetic resonance spectroscopy of Alzheimer disease. Alzheimer Dis Assoc Disord 10(1):46–52
Hachinski VC, Iliff LD, Phil M, Zilhka E, Du Boullay GH, Mcallister VI et al (1975) Cerebral blood flow in dementia. Arch Neurol 32:632–637
Hodkinson HM (1972) Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1:233–238
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572
Klein J (2000) Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm 107(8–9):1027–1063
Lukiw WJ, Bazan NG (2000) Neuroinflammatory signaling upregulation in Alzheimer’s disease. Neurochem Res 25(9–10):1173–1184
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7):939–944
Mulder C, Wahlund LO, Teerlink T, Blomberg M, Veerhuis R, Van Kamp GJ et al (2003) Decreased lysophosphatidylcholine/phosphatidylcholine ratio in cerebrospinal fluid in Alzheimer’s disease. J Neural Transm 110(8):949–955
Murphy DG, Bottomley PA, Salerno JA, DeCarli C, Mentis MJ, Grady CL et al (1993) An in vivo study of phosphorus and glucose metabolism in Alzheimer’s disease using magnetic resonance spectroscopy and PET. Arch Gen Psychiatry 50(5):341–349
Ordidge RJ, Connely A, Lohman JÁ (1986) Image-selected in vivo spectroscopy (ISIS). A new technique for spatially selective NMR spectroscopy. J Magn Reson 66:283–294
Pettegrew JW, Kanagasabai P, Moossy J, Martinez J, Rao G, Boller F (1988) Correlation of phosphorus-31 magnetic resonance spectroscopy and morphologic findings in Alzheimer’s disease. Arch Neurol 45:1093–1096
Pettegrew JW, Panchalingam K, Klunk WE, McClure RJ, Muenz LR (1994) Alterations of cerebral metabolism in probable Alzheimer’s disease: a preliminary study. Neurobiol Aging 15(1):117–132
Pettegrew JW, Klunk WE, Kanal E, Panchalingam K, McClure RJ (1995) Changes in brain membrane phospholipid and high-energy phosphate metabolism precede dementia. Neurobiol Aging 16(6):973–975
Ross BM, Moszczynska A, Erlich J, Kish SJ (1998) Phospholipid-metabolizing enzymes in Alzheimer’s disease: increased lysophospholipid acyltransferase activity and decreased phospholipase A2 activity. J Neurochem 70(2):786–793
Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, Goddard R (1986) CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 149:698–709
Schaeffer E, Gattaz WF (in press) Inhibition of calcium-independent phospholipase A2 activity in rat hippocampus impairs short- and long-term memory formation. Psychopharmacology
Skinner ER, Watt C, Besson JAO, Best PV (1989) Lipid composition of different regions of the brain in patients with Alzheimer’s disease. Biochem Soc Trans 17:213–214
Smith CD, Gallenstein LG, Layton WJ, Kryscio RJ, Markesbery WR (1993) 31P magnetic resonance spectroscopy in Alzheimer’s and Pick’s disease. Neurobiol Aging 14(1):85–92
Smith CD, Pettigrew LC, Avison MJ, Kirsch JE, Tinkhtman AJ, Schmitt FA et al (1995) Frontal lobe phosphorus metabolism and neuropsychological function in aging and in Alzheimer’s disease. Ann Neurol 38(2):194–201
Stephenson DT, Manetta JV, White DL, Chiou XG, Cox L, Gitter B, May PC, Sharp JD, Kramer RM, Clemens JA (1994) Calcium-sensitive cytosolic phospholipase A2 (cPLA2) is expressed in human brain astrocytes. Brain Res 637:97–105
Talbot K, Young RA, Jolly-Tornetta C, Lee VM, Trojanowski JQ, Wolf BA (2000) A frontal variant of Alzheimer’s disease exhibits decreased calcium-independent phospholipase A2 activity in the prefrontal cortex. Neurochem Int 37(1):17–31
Vanhamme L, van den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129(1):35–43
Yacubian J, de Castro CC, Ometto M, Barbosa E, de Camargo CP, Tavares H, Cerri GG, Gattaz WF (2002) 31P-spectroscopy of frontal lobe in schizophrenia: alterations in phospholipid and high-energy phosphate metabolism. Schizophr Res 58(2–3):117–122
Acknowledgements
The present study was supported by Fundação do Amparo à Pesquisa do Estado de São Paulo (FAPESP, Projects 97/11083-0, 99/00740-5, and 02/13633-7). The Laboratory of Neuroscience receives financial support from the Associação Beneficente Alzira Denise Herzog da Silva (ABADHS).
We are indebted to Dr. Eduardo Simão, M.D., for the contribution to data collection and conduction of MRS scans.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forlenza, O.V., Wacker, P., Nunes, P.V. et al. Reduced phospholipid breakdown in Alzheimer’s brains: a 31P spectroscopy study. Psychopharmacology 180, 359–365 (2005). https://doi.org/10.1007/s00213-005-2168-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2168-8