Abstract
Introduction
Cannabis users have been reported to have decreased regional cerebral glucose metabolism after short periods of abstinence. The purpose of this study was to measure striatal dopamine receptor (D2/D3) availability and cerebral glucose metabolism with positron emission tomography (PET) in young adults who had a prolonged exposure to cannabis and who had been abstinent for a period of at least 12 weeks.
Materials and methods
Six 18–21-year-old male subjects with cannabis dependence in early full remission and six age- and sex-matched healthy subjects underwent PET scans for D2/D3 receptor availability measured with [C11]-raclopride and glucose metabolism measured with [18F]-FDG. All subjects were sober for at least 12 weeks before PET scan procedures. PET data were analyzed with statistical parametric mapping software (SPM99; uncorrected p < 0.001, corrected p < 0.05 at the cluster level). Toxicology screening was performed prior to the PET scan to confirm the lack of drugs of abuse.
Observation and results
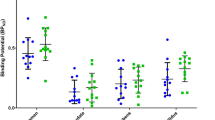
Striatal D2/D3 receptor availability did not differ significantly between groups. Compared to controls, subjects with cannabis dependence had lower normalized glucose metabolism in the right orbitofrontal cortex, putamen bilaterally, and precuneus. There were no significant correlations between striatal D2/D3 receptor availability and normalized glucose metabolism in any region of the frontal cortex or striatum.
Conclusion
These findings may reflect both cannabis exposure and adaptive changes that occur after a prolonged period of abstinence. Subsequent studies should address whether metabolic and dopamine receptor effects are associated with either active use or longer-term withdrawal in these relatively young subjects.

Similar content being viewed by others
References
Amen DG, Waugh M (1998) High resolution brain SPECT imaging of marijuana smokers with AD/HD. J Psychoactive Drugs 30:209–214
Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, Bressman SB, Eidelberg D (2005) Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology 64:347–349
Block RI, O'Leary DS, Hichwa RD, Augustinack JC, Ponto LL, Ghoneim MM, Arndt S, Ehrhardt JC, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC (2000) Cerebellar hypoactivity in frequent marijuana users. Neuroreport 11:749–753
Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR Jr., Madsen D, Jarvik ME (2002) Brain metabolic changes during cigarette craving. Arch Gen Psychiatry 59:1162–1172
Chaly T, Mattacchieri R, Velez JW, Dahl JR, Margouleff D (1990) A large scale production of 18F-FDG using a synthetic unit made of sterile disposable components and operated by a slave manipulator. Appl Radiat Isot 41:29–34
Chang L, Yakupov R, Cloak C, Ernst T (2006) Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 129:1096–1112
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. LEA, Mahwah
Collins DL, Neelin P, Peters TM, Evans AC (1994) Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205
Dhawan V, Kazumata K, Robeson W, Belakhlef A, Margouleff C, Chaly T, Nakamura T, Dahl R, Margouleff D, Eidelberg D (1998) Quantitative Brain PET. Comparison of 2D and 3D Acquisitions on the GE Advance Scanner. Clin Positron Imaging 1:135–144
Farde L, Pauli S, Hall H, Eriksson L, Halldin C, Hogberg T, Nilsson L, Sjogren I, Stone-Elander S (1988) Stereoselective binding of 11C-raclopride in living human brain—a search for extrastriatal central D2-dopamine receptors by PET. Psychopharmacology (Berl) 94:471–478
Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ (1995) Statistical parametric maps in functional imaging: a general linear approach. Human Bran Mapping 2:189–210
Gardner EL (2005) Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav 81:263–284
Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D (1999) Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2:358–363
Glass M, Dragunow M, Faull RL (1997) Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77:299–318
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87:1932–1936
Kalivas P (2002) Neurocircuitry of addiction. In: Davis K, Charney D, Coyle J, Nemeroff C (eds) Neuropsychopharmacology: The Fifth Generation of Progress. ACNP, Nahville, pp 1357–1366
Koob GF, Bloom FE (1988) Cellular and molecular mechanisms of drug dependence. Science 242:715–723
Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F (1998) Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res 22:3–9
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RS (1996) Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab 16:42–52
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840
Lundqvist T, Jonsson S, Warkentin S (2001) Frontal lobe dysfunction in long-term cannabis users. Neurotoxicol Teratol 23:437–443
Ma Y, Smith G, Dhawan V, Chaly T, Pollock B, Eidelberg D (2002) Selection of reference regions in the analysis of D2 receptor binding data with [11C]raclopride: cerebellum versus occipital cortex. NeuroImage 16:S46
Mailleux P, Vanderhaeghen JJ (1992) Localization of cannabinoid receptor in the human developing and adult basal ganglia. Higher levels in the striatonigral neurons. Neurosci Lett 148:173–176
Meyers K, McLellan AT, Jaeger JL, Pettinati HM (1995) The development of the Comprehensive Addiction Severity Index for Adolescents (CASI-A). An interview for assessing multiple problems of adolescents. J Subst Abuse Treat 12:181–193
Nelson HE, O'Connell A (1978) Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex 14:234–244
Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ (1991) Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 48:648–654
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE (1979) Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 6:371–388
SAMHSA (2006) Results from the 2005 National Survey on Drug Use and Health: National Findings. Office of Applied Studies, Rockville
Smith GS, Ma Y, Dhawan V, Gunduz H, Carbon M, Kirshner M, Larson J, Chaly T, Belakhleff A, Kramer E, Greenwald B, Kane JM, Laghrissi-Thode F, Pollock BG, Eidelber D (2002) Serotonin modulation of cerebral glucose metabolism measured with positron emission tomography (PET) in human subjects. Synapse 45:105–512
Takikawa S, Dhawan V, Spetsieris P, Robeson W, Chaly T, Dahl R, Margouleff D, Eidelberg D (1993) Noninvasive quantitative fluorodeoxyglucose PET studies with an estimated input function derived from a population-based arterial blood curve. Radiology 188:131–136
Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N (2001) Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158:2015–2021
Volkow ND, Fowler JS (2000) Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 10:318–325
Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP (1993) Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14:169–177
Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L (1996a) Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res 67:29–38
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N (1997) Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386:830–833
Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K (1996b) Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 20:1594–1598
Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N (2002) Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse 46:79–82
Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C (1999) Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 64:775–784
Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K (1997) Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology 16:174–182
Wise RA, Bozarth MA (1985) Brain mechanisms of drug reward and euphoria. Psychiatr Med 3:445–460
Acknowledgements
This work was supported by the National Institute Health, grants K23 DA015541 (SS), K02 MH01621, MH 64823 (GS), M01 RR018535, and a Faculty Award from the Feinstein Institute for Medical Research (Manhasset, NY) (SS). David Bjelke, CNMT and Claude Margouleff, B.S. are gratefully acknowledged for their contribution to the conduct of the PET studies. We thank Terry Goldberg, PhD for his review of the manuscript, Steve Grant, PhD of the National Institute on Drug Abuse and Barbara Napolitano, MS for their helpful comments.
Financial disclosures
Drs. Sevy, Smith, Ma, Dhawan, Chaly, Kingsley, Kumra, Mr. Abdelmessih, and Dr. Eidelberg reported no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sevy, S., Smith, G.S., Ma, Y. et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology 197, 549–556 (2008). https://doi.org/10.1007/s00213-008-1075-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1075-1