Abstract
Rationale
Tramadol is an atypical, mixed-mechanism analgesic involving both opioid and catecholamine processes that appears to have low abuse potential and may be useful as a treatment for opioid dependence.
Objectives
The current study assessed the level of physical dependence and opioid blockade efficacy produced by daily maintenance on oral tramadol.
Methods
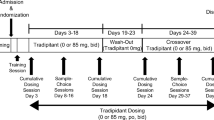
Nine residential opioid-dependent adults were maintained on two doses of daily oral tramadol (200 and 800 mg) for approximately 4-week intervals in a randomized, double-blind, crossover design. The acute effects of intramuscular placebo, naloxone (0.25, 0.5, and 1.0 mg), and hydromorphone (1.5, 3.0, and 6.0 mg) were tested under double-blind, randomized conditions. Outcomes included observer- and subject-rated measures and physiologic indices.
Results
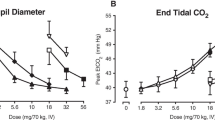
Challenge doses of naloxone resulted in significantly higher mean peak withdrawal scores compared to placebo. Withdrawal intensity from naloxone was generally greater during 800 versus 200 mg/day tramadol maintenance. Mean peak ratings of agonist effects were elevated at higher hydromorphone challenge doses, but did not differ significantly between tramadol doses. Physiologic measures were generally affected by challenge conditions in a dose-dependent manner, with few differences between tramadol maintenance dose conditions.
Conclusions
Chronic tramadol administration produces dose-related opioid physical dependence, without producing dose-related attenuation of agonist challenge effects. Tramadol may be a useful treatment for patients with low levels of opioid dependence or as a treatment for withdrawal during opioid detoxification, but does not appear to be effective as a maintenance medication due to a lack of opioid cross-tolerance.


Similar content being viewed by others
References
Barsotti CE, Mycyk MB, Reyes J (2003) Withdrawal syndrome from tramadol hydrochloride. Am J Emerg Med 21:87–88
Cami J, Lamas X, Farre M (1994) Acute effects of tramadol in methadone-maintained volunteers. Drugs 47(Suppl 1):39–43
Carroll CP, Walsh SL, Bigelow GE, Strain EC, Preston KL (2006) Assessment of agonist and antagonist effects of tramadol in opioid-dependent humans. Exp Clin Psychopharmacol 14:109–120
Cicero TJ, Adams EH, Geller A, Inciardi JA, Munoz A, Schnoll SH, Senay EC, Woody GE (1999) A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend 57:7–22
Cicero TJ, Inciardi JA, Adams EH, Geller A, Senay EC, Woody GE, Munoz A (2005) Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994–2004. Pharmacoepidemiol Drug Saf 14:851–859
Desmeules JA, Piguet V, Collart L, Dayer P (1996) Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol 41:7–12
Driessen B, Reimann W, Giertz H (1993) Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro. Br J Pharmacol 108:806–811
Ehrenreich H, Poser W (1993) Dependence on tramadol. Clin Investig 72:76
Epstein DH, Preston KL, Jasinski DR (2006) Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: lessons from tramadol. Biol Psychol 73:90–99
Freye E, Levy J (2000) Acute abstinence syndrome following abrupt cessation of long-term use of tramadol (Ultram): a case study. Eur J Pain 4:307–311
Friderichs E, Felgenhauer F, Jongschaap P, Osterloh G (1978) Pharmacological studies on analgesia, dependence on and tolerance of tramadol, a potent analgetic drug (author’s transl). Arzneimittelforschung 28:122–134
Frink M, Hennies HH, Englberger W, Haurand M, Wilffert B (1996) Influence of tramadol on neurotransmitter systems of the rat brain. Arzneimittelforschung 46:1029–1036
Gillen C, Haurand M, Kobelt DJ, Wnendt S (2000) Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol 362:116–121
Gutstein H, Akil H (2001) Opioid Analgesics. In: Hardman J, Limbird L (eds) Goodman & Gilman’s the pharmacological basis of therapeutics, 10th edn. McGraw-Hill, New York, pp 569–619
Inciardi JA, Cicero TJ, Munoz A, Adams EH, Geller A, Senay EC, Woody GE (2006) The diversion of Ultram, Ultracet, and generic tramadol HCL. J Addict Dis 25:53–58
Jasinski DR, Preston KL, Sullivan JT, Testa M (1993) Abuse potential of oral tramadol. NIDA Res Monogr 132:103
Kayser V, Besson JM, Guilbaud G (1992) Evidence for a noradrenergic component in the antinociceptive effect of the analgesic agent tramadol in an animal model of clinical pain, the arthritic rat. Eur J Pharmacol 224:83–88
Lofwall MR, Walsh SL, Bigelow GE, Strain EC (2007) Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology (Berl) 194:381–393
Miranda HF, Pinardi G (1998) Antinociception, tolerance, and physical dependence comparison between morphine and tramadol. Pharmacol Biochem Behav 61:357–360
Murano T, Yamamoto H, Endo N, Kudo Y, Okada N, Masuda Y, Yano I (1978) Studies on dependence on tramadol in rats. Arzneimittelforschung 28:152–158
Preston KL, Jasinski DR (1989) Effects of tramadol in humans: assessment of its abuse potential. NIDA Res Monogr 95:392
Preston KL, Jasinski DR, Testa M (1991) Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend 27:7–17
Raffa RB (2008) Basic pharmacology relevant to drug abuse assessment: tramadol as an example. J Clin Pharm Ther 33:101–108
Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL (1992) Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 260:275–285
Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL, Jacoby HI, Selve N (1993) Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther 267:331–340
Richter W, Barth H, Flohe L, Giertz H (1985) Clinical investigation on the development of dependence during oral therapy with tramadol. Arzneimittelforschung 35:1742–1744
Salehi M, Amanatkar M, Barekatain M (2005) Tramadol versus methadone for the management of acute opioid withdrawal: an add-on study. J Res Med Sci 11:185–189
Senay EC, Adams EH, Geller A, Inciardi JA, Munoz A, Schnoll SH, Woody GE, Cicero TJ (2003) Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend 69:233–241
Sobey PW, Parran TV Jr, Grey SF, Adelman CL, Yu J (2003) The use of tramadol for acute heroin withdrawal: a comparison to clonidine. J Addict Dis 22:13–25
Spitzer RL, Williams JB, Gibbon M, First MB (1992) The structured clinical interview for DSM-III-R (SCID). I. History, rationale, and description. Arch Gen Psychiatry 49:624–629
Strain EC, Stoller K, Walsh SL, Bigelow GE (2000) Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology (Berl) 148:374–383
Tamaskar R, Parran TV Jr, Heggi A, Brateanu A, Rabb M, Yu J (2003) Tramadol versus buprenorphine for the treatment of opiate withdrawal: a retrospective cohort control study. J Addict Dis 22:5–12
Threlkeld M, Parran TV, Adelman CA, Grey SF, Yu J (2006) Tramadol versus buprenorphine for the management of acute heroin withdrawal: a retrospective matched cohort controlled study. Am J Addict 15:186–191
Woody GE, Senay EC, Geller A, Adams EH, Inciardi JA, Schnoll S, Munoz A, Cicero TJ (2003) An independent assessment of MEDWatch reporting for abuse/dependence and withdrawal from Ultram (tramadol hydrochloride). Drug Alcohol Depend 72:163–168
Yanagita T (1978) Drug dependence potential of 1-(m-methoxyphenyl)-2-dimethylaminomethyl)-cyclohexan-1-ol hydrochloride (tramadol) tested in monkeys. Arzneimittelforschung 28:158–163
Yates WR, Nguyen MH, Warnock JK (2001) Tramadol dependence with no history of substance abuse. Am J Psychiatry 158:964
Zacny JP (2005) Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend 80:273–278
Acknowledgments
The authors thank Elliot Joseph, Jessica Vanderhoff, Sonia Bansal, Mary Misenhimer, Sarah Ilk, John Yingling, Linda Felch, and the nursing staff at the Behavioral Pharmacology Research Unit for their assistance in volunteer recruitment, data collection, and analysis. This study complies with current laws of the USA.
Disclosure
Tramadol was developed by Grünenthal. Dr. Strain is a paid consultant to Grünenthal. This arrangement is being managed by the Johns Hopkins University in accordance with its conflict of interest policies. In recent years, Dr. Bigelow has received consulting payments from Abbott Laboratories, Takeda Pharmaceuticals, and Teva Pharmaceuticals and through his university has received research support from Titan Pharmaceuticals and Pain Therapeutics, Inc. Dr. Lanier is now an employee of Rock Creek Pharmaceuticals.
Funding
This work was supported by the National Institutes of Health/National Institute on Drug Abuse Grant R01DA018125 to Johns Hopkins University (ECS), Midcareer Investigator Award in Patient-Oriented Research K24D023186 (ECS), and Training Grant T32DA07209 to Johns Hopkins University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lanier, R.K., Lofwall, M.R., Mintzer, M.Z. et al. Physical dependence potential of daily tramadol dosing in humans. Psychopharmacology 211, 457–466 (2010). https://doi.org/10.1007/s00213-010-1919-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1919-3