Abstract
Rationale
Methamphetamine (METH) is a powerfully addictive stimulant associated with serious health conditions. Accumulating evidence suggests a role of oxidative stress in METH-induced behavioral abnormalities. Sulforaphane (SFN), found in cruciferous vegetables, is a potent antioxidant. It is of interest to determine whether SFN can attenuate behavioral and neuropathological changes associated with METH exposure.
Objectives
This study was undertaken to examine the effects of SFN on behavioral changes and dopaminergic neurotoxicity in mice exposed to METH.
Methods
The effects of SFN on acute hyperlocomotion and the development of behavioral sensitization induced by the administration of METH were examined. Levels of dopamine (DA) and its major metabolite 3,4-dihydroxyphenyl acetic acid (DOPAC) in the striatum were measured. In addition, DA transporter (DAT) immunoreactivity was also performed.
Results
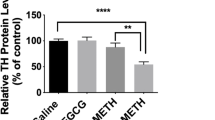
Pretreatment with SFN at 1, 3, and 10 mg/kg elicited a dose-dependent attenuation of acute hyperlocomotion in mice, after a single administration of METH (3 mg/kg). The development of behavioral sensitization after repeated administrations of METH (3 mg/kg/day, once daily for 5 days) was significantly reduced by pretreatment with SFN (10 mg/kg). In addition, the lowering of DA levels and DOPAC as well as DAT immunoreactivity in the striatum, usually seen after repeated administration of METH, was significantly attenuated by both pretreatment and the subsequent administration of SFN. Furthermore, SFN significantly reduced microglial activation in the striatum after repeated exposure to METH.
Conclusion
It is therefore likely that SFN can be a useful drug for the treatment of signs associated with METH abuse in humans.






Similar content being viewed by others
Abbreviations
- METH:
-
Methamphetamine
- SFN:
-
Sulforaphane
- DA:
-
Dopamine
- DOPAC:
-
3,4-Dihydroxyphenyl acetic acid
- DAT:
-
Dopamine transporter
- PET:
-
Positron emission tomography
- Nrf2:
-
NF-E2-related factor-2
- ARE:
-
Antioxidant responsive element
- HPLC:
-
High performance liquid chromatography
References
Açikgöz O, Gonenc S, Gezer S et al (2001) Methamphetamine causes depletion of glutathione and an increase in oxidized glutathione in the rat striatum and prefrontal cortex. Neurotox Res 3:277–280
Albers DS, Sonsalla PK (1995) Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther 275:1104–1114
Ali SF, Newport GD, Slikker W Jr (1996) Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann NY Acad Sci 801:187–198
Barr A, Panenka W, MacEwan W et al (2006) The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci 31:301–313
Cadet JL, Jayanthi S, Deng X (2003) Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J 17:1775–1788
Cadet JL, Krasnova IN, Jayanthi S, Lyles J (2007) Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res 11:183–202
Chen HX, Wu J, Zhang JC, Hashimoto K (2010) Recent topics on pharmacotherapy for amphetamine-type stimulants abuse and dependence. Curr Drug Abuse Rev 3:222–238
Cheung KL, Kong AN (2010) Molecular targets of dietary phenylethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J 12:87–97
Colfax G, Santos GM, Chu P et al (2010) Amphetamine-group substances and HIV. Lancet 376:458–474
Danilov CA, Chandrasekaran K, Racz J, Soane L, Zielke C, Fiskum G (2009) Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia 57:645–656
Davidson C, Gow AJ, Lee TH, Ellinwood EH (2001) Methamphetamine neutoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev 6:1–22
Escubedo E, Guitart L, Sureda FX et al (1998) Microgliosis and down-regulation of adenosine transporter induced by methamphetamine in rats. Brain Res 814:120–126
Fenwick GR, Heaney RK, Mullin WJ (1983) Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr 18:123–201
Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M (2004) Effect of antioxidant N-acetyl-l-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res 1016:90–95
Gonzales R, Mooney L, Rawson RA (2010) The methamphetamine problem in the United States. Annu Rev Public Health 31:385–398
Guilarte TR, Nihei MK, McGlothan JL, Howard AS (2003) Methamphetamine induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience 122:499–513
Hagiwara H, Iyo M, Hashimoto K (2009) Mithramycin protects against dopaminergic neurotoxicity in the mouse brain after administration of methamphetamine. Brain Res 1301:189–196
Han JM, Lee YJ, Lee SY et al (2007) Protective effect of sulforaphane against dopaminergic cell death. J Pharmacol Exp Ther 321:249–256
Hashimoto K (2007) New research on methamphetamine abuse. In: Toolaney GH (ed) New research on methamphetamine abuse. NovaScience, New York, pp 1–51
Hashimoto K, Tsukada H, Nishiyama S et al (2004) Protective effects of N-acetyl-l-cysteine on the reduction of dopamine transporters in the striatum of monkeys treated with methamphetamine. Neuropsychopharmacology 29:2018–2023
Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Iyo M (2007) Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a PET study in conscious monkeys. Biol Psychiatry 61:577–581
Innamorato NG, Rojo AI, Garcia-Yagüe AJ, Yamamoto M, de Ceballos ML, Cuadrado A (2008) The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol 181:680–689
Itoh K, Tong KI, Yamamoto M (2004) Molecular mechanism activating Nrf2–Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36:1208–1213
Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernández-Ruiz J, Cuadrado A (2011) Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal 14:2347–2360
Juge N, Mithen RF, Traka M (2007) Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci 64:1105–1127
Kang KW, Lee SJ, Kim SG (2005) Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal 7:1664–1673
Kita T, Paku S, Takahashi M, Kubo K, Wagner GC, Nakashima T (1998) Methamphetamine-induced neurotoxicity in BALB/c, DBA/2 N and C57BL/6 N mice. Neuropharmacology 37:1177–1784
Koike K, Hashimoto K, Fukami G et al (2005) The immunophilin ligand FK506 protects against methamphetamine-induced dopaminergic neurotoxicity in mouse striatum. Neuropharmacology 48:391–397
Kwak MK, Kensler TW (2010) Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol 244:66–76
LaVoie MJ, Card JP, Hastings TG (2004) Microglia activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol 187:47–57
Licata SC, Pierce RC (2003) The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J Neurochem 85:14–22
Miyazaki I, Asanuma M, Diaz-Corrales FJ et al (2006) Methamphetamine-induced dopaminergic neurotoxicity is regulated by quinone formation-related molecules. FASEB J 20:571–573
National Institute on Drug Abuse (2002) Research report series—methamphetamine abuse and addiction. NIDA, Rockville
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev 25:192–216
Ping Z, Liu W, Kang Z et al (2010) Sulforaphane protects brains against hypoxic–ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res 1343:178–185
Pubill D, Canudas AM, Pallas M, Camins A, Camarasa J, Escubedo E (2003) Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn Schmiedebergs Arch Pharmacol 367:490–499
Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res 396:157–198
Scholl JL, Feng N, Watt MJ, Renner KJ, Forster GL (2009) Individual differences in amphetamine sensitization, behavior and central monoamines. Physiol Behav 96:493–504
Seiden LS, Sabol KE, Ricaurte GA (1993) Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol 33:639–677
Sekine Y, Iyo M, Ouchi Y et al (2001) Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry 158:1206–1214
Siebert A, Desai V, Chandrasekaran K, Fiskum G, Jafri MS (2009) Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res 87:1659–1669
Thomas DM, Kuhn DM (2005) Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem 92:790–797
Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM (2004) Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglia activation. J Pharmacol Exp Ther 311:1–7
Ujike H, Sato M (2004) Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann NY Acad Sci 1025:279–287
United Nations Office on Drug Use and Crime (UNODC) (2008) World drug report. UNODC, Vienna
Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99–120
Volkow ND, Chang L, Wang GJ et al (2001) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377–382
White FJ, Kalivas PW (1998) Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend 51:141–153
Wilson JM, Kalansinsky KS, Levey AI et al (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703
Yamamoto J (2004) Recent trends of drug abuse in Japan. Ann NY Acad Sci 1025:430–438
Yamamoto BK, Raudensky J (2008) The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol 3:203–217
Yanaka A (2011) Sulforaphane enhances protection and repair of gastric mucosa against oxidative stress in vitro, and demonstrates anti-inflammatory effects on Helicobacter pylori-infected gastric mucosae in mice and human subjects. Curr Pham Des 17:1532–1540
Yanaka A, Fahey JW, Fukumoto A et al (2009) Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) 2:353–260
Zhang Y, Li J, Tang L (2005) Cancer-preventive isothiocyanates: dichotomous modulators of oxidative stress. Free Radic Biol Med 38:70–77
Zhang L, Kitaichi K, Fujimoto Y et al (2006) Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuro-Psychopharmacol Biol Psychiatry 30:1381–1393
Acknowledgment
This study is supported partly by a grant from Intramural Research Grant (22-2: to K.H.) for Neurological and Psychiatric Disorders of NCNP, Japan.
Conflicts of interest
All authors had no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Effects of SFN on hyperlocomotion in mice after the three administration of METH. Thirty minutes after i.p. injection of vehicle (10 ml/kg) or SFN (10 mg/kg), mice received three injections of METH (3 mg/kg, s.c.) or vehicle (10 ml/kg, s.c.) at 3-h intervals. Behavior (locomotion) in the mice was evaluated. Each value is the mean ± SEM (n = 7 or 8 per group). *p < 0.05, **p < 0.01, ***p < 0.001 as compared with the vehicle + METH group (Bonferroni/Dunn method) (JPEG 23 kb)
Rights and permissions
About this article
Cite this article
Chen, H., Wu, J., Zhang, J. et al. Protective effects of the antioxidant sulforaphane on behavioral changes and neurotoxicity in mice after the administration of methamphetamine. Psychopharmacology 222, 37–45 (2012). https://doi.org/10.1007/s00213-011-2619-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2619-3