Abstract
Introduction
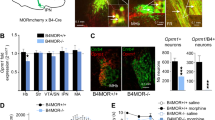
Agonists at the mu opioid receptor (MOR) are widely recognized for their effects on reward and pain. Although prior studies have attributed some of these effects to MORs on GABA neurons in the ventral tegmental area (VTA), recent studies have identified a region of particularly strong MOR immunostaining residing caudal to the VTA, in a region denoted the rostromedial tegmental nucleus (RMTg).
Methods
Hence, we examined whether rats would self-administer small doses (50–250 pmol) of the selective MOR agonist endomorphin-1 (EM1) into the RMTg and adjacent sites. EM1 was chosen due to its short half-life, thus limiting drug spread, and due to its presence endogenously in brain neurons, including some afferents to the RMTg.
Results
The highest rates of EM1 self-administration occurred within 0.5 mm of the RMTg center, in a region roughly 0.8–1.6 mm caudal to the majority of VTA DA neurons. In contrast, self-administration rates were much lower in the adjacent VTA, interpeduncular nucleus, central linear nucleus, or median raphe nucleus. Furthermore, EM1 infusions into the RMTg, but not surrounding regions, produced conditioned place preference, while EM1 infusions into the RMTg but not anterior VTA markedly reduced formalin-induced pain behaviors. EM1 effects were mimicked by infusions of the GABA agonist muscimol into the same region, consistent with EM1 having inhibitory actions on its target neurons.
Conclusion
These results implicate a novel brain region in modulating MOR influences on both appetitive and aversive behavior.





Similar content being viewed by others
References
Altier N, Stewart J (1998) Dopamine receptor antagonists in the nucleus accumbens attenuate analgesia induced by ventral tegmental area substance P or morphine and by nucleus accumbens amphetamine. J Pharmacol Exp Ther 285:208–215
Altier N, Stewart J (1999) The role of dopamine in the nucleus accumbens in analgesia. Life Sci 65:2269–2287
Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR (2011) The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol 519:1143–1164
Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS (1993) Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264:489–495
Basbaum AI, Fields HL (1984) Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7:309–338
Bozarth MA, Wise RA (1981a) Heroin reward is dependent on a dopaminergic substrate. Life Sci 29:1881–1886
Bozarth MA, Wise RA (1981b) Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci 28:551–555
Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE (2001) Orexin (hypocretin) neurons contain dynorphin. J Neurosci 21: RC168
Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB (2002) Afferents to the ventrolateral preoptic nucleus. J Neurosci 22:977–990
Cohen SR, Melzack R (1985) Morphine injected into the habenula and dorsal posteromedial thalamus produces analgesia in the formalin test. Brain Res 359:131–139
David V, Durkin TP, Cazala P (2002) Differential effects of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology (Berl) 160:307–317
Devine DP, Wise RA (1994) Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci 14:1978–1984
Ding YQ, Kaneko T, Nomura S, Mizuno N (1996) Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol 367:375–402
Dubuisson D, Dennis SG (1977) The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4:161–174
Fichna J, Janecka A, Piestrzeniewicz M, Costentin J, do Rego JC (2007) Antidepressant-like effect of endomorphin-1 and endomorphin-2 in mice. Neuropsychopharmacol 32:813–821
Greco MA, Fuller PM, Jhou TC, Martin-Schild S, Zadina JE, Hu Z, Shiromani P, Lu J (2008) Opioidergic projections to sleep-active neurons in the ventrolateral preoptic nucleus. Brain Res 1245:96–107
Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O (2011) Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J. Neurosci
Houdi AA, Kottayil S, Crooks PA, Butterfield DA (1996) 3-O-acetylmorphine-6-O-sulfate: a potent, centrally acting morphine derivative. Pharmacol Biochem Behav 53:665–671
Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56:27–78
Ikemoto S, Donahue KM (2005) A five-minute, but not a fifteen-minute, conditioning trial duration induces conditioned place preference for cocaine administration into the olfactory tubercle. Synapse 56:57–59
Ikemoto S, Sharpe LG (2001) A head-attachable device for injecting nanoliter volumes of drug solutions into brain sites of freely moving rats. J Neurosci Methods 110:135–140
Ikemoto S, Murphy JM, McBride WJ (1998) Regional differences within the rat ventral tegmental area for muscimol self-infusions. Pharmacol Biochem Behav 61:87–92
Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F (2011) Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A 108:16446–16450
Jhou T (2005) Neural mechanisms of freezing and passive aversive behaviors. J Comp Neurol 493:111–114
Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC (2009a) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61:786–800
Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS (2009b) The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol 513:566–596
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M (2009) Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol 513:597–621
Lavezzi HN, Zahm DS (2011) The mesopontine rostromedial tegmental nucleus: an integrative modulator of the reward system. Basal Ganglia 1:191–200
Lavezzi HN, Parsley KP, Zahm DS (2011) Mesopontine rostromedial tegmental nucleus neurons projecting to the dorsal raphe and pedunculopontine tegmental nucleus: psychostimulant-elicited Fos expression and collateralization. Brain Struct Funct
Le Merrer J, Becker JA, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412
Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M (2011a) Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology 36:589–602
Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M (2011b) Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology
Manning BH, Franklin KB (1998) Morphine analgesia in the formalin test: reversal by microinjection of quaternary naloxone into the posterior hypothalamic area or periaqueductal gray. Behav Brain Res 92:97–102
Manning BH, Morgan MJ, Franklin KB (1994) Morphine analgesia in the formalin test: evidence for forebrain and midbrain sites of action. Neurosci 63:289–294
Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ (1994) Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 350:412–438
Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE (1999) Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405:450–471
Matsui A, Williams JT (2011) Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci 31:17729–17735
Metzger M, Goncalves L, Sego C (2010) Immunohistochemical and topographical characterization of the rostromedial tegmental nucleus. Soc Neurosci Abstr 916.25
Morales M, Wang H, Zhang P, Ikemoto S, Jhou TC (2011) Expression of prepronociceptin in the rostromedial tegmentum (RMTg). Soc Neurosci Abstr
Morgan MJ, Franklin KB (1991) Dopamine receptor subtypes and formalin test analgesia. Pharmacol Biochem Behav 40:317–322
Nader K, van der Kooy D (1997) Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci 17:383–390
Negus SS, Henriksen SJ, Mattox A, Pasternak GW, Portoghese PS, Takemori AE, Weinger MB, Koob GF (1993) Effect of antagonists selective for mu, delta and kappa opioid receptors on the reinforcing effects of heroin in rats. J Pharmacol Exp Ther 265:1245–1252
Olmstead MC, Franklin KB (1997) The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci 111:1324–1434
Perlikowska R, Gach K, Fichna J, Toth G, Walkowiak B, do-Rego JC, Janecka A (2009) Biological activity of endomorphin and [Dmt1]endomorphin analogs with six-membered proline surrogates in position 2. Bioorg Med Chem 17:3789–3794
Shippenberg TS, Bals-Kubik R, Herz A (1993) Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther 265:53–59
Simmons DM, Arriza JL, Swanson LW (1989) A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radio-labeled single-stranded RNA probes. J Histotechnol 3:169–181
Terashvili M, Wu HE, Leitermann RJ, Hung KC, Clithero AD, Schwasinger ET, Tseng LF (2004) Differential conditioned place preference responses to endomorphin-1 and endomorphin-2 microinjected into the posterior nucleus accumbens shell and ventral tegmental area in the rat. J Pharmacol Exp Ther 309:816–824
Vargas-Perez H, Ting AKR, van der Kooy D (2009) Different neural systems mediate morphine reward and its spontaneous withdrawal aversion. Eur J Neurosci 29:2029–2034
Welzl H, Kuhn G, Huston JP (1989) Self-administration of small amounts of morphine through glass micropipettes into the ventral tegmental area of the rat. Neuropharmacology 28:1017–1023
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
Yaksh TL, Yeung JC, Rudy TA (1976) Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res 114:83–103
Zangen A, Ikemoto S, Zadina JE, Wise RA (2002) Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci 22:7225–7233
Acknowledgments
We acknowledge Toni Shippenberg and Vicky Minney for helpful technical advice. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Conflict of interest
The authors declare no competing financial interests with the work described in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jhou, T.C., Xu, SP., Lee, M.R. et al. Mapping of reinforcing and analgesic effects of the mu opioid agonist Endomorphin-1 in the ventral midbrain of the rat. Psychopharmacology 224, 303–312 (2012). https://doi.org/10.1007/s00213-012-2753-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2753-6