Abstract
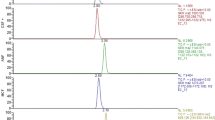
A semi-automated liquid chromatography–tandem mass spectrometry (LC/MS/MS) method was developed for the simultaneous quantification of the antifungal drug itraconazole (ITZ) and its coactive metabolite hydroxyitraconazole (OH-ITZ) in human plasma. The plasma samples underwent liquid–liquid extraction (LLE) in 2.2 mL 96 deepwell plates. ITZ, OH-ITZ and the internal standard (IS) R51012 were extracted from plasma, using a mixture of acetonitrile (ACN) and methyl t-butyl ether (MTBE) as the organic solvent. This specific mixture, due to its composition, had a significant impact on the performance of the assay. All liquid transfer steps, including preparation of calibration standards and quality control samples as well as the addition of the IS, were performed automatically using robotic liquid handling workstations for parallel sample processing. After vortexing, centrifugation and freezing, the supernatant organic solvent was evaporated. The analytes and IS were dissolved in a small volume of a reconstitution solution, an aliquot of which was analyzed by combined reversed phase LC/MS/MS, with positive ion electrospray ionization and a TurboIonSpray interface, using multiple reactions monitoring (MRM). The method was shown to be sensitive and specific to both ITZ and OH-ITZ, it revealed excellent linearity for the range of concentrations 2–500 ng mL−1 for ITZ and 4–1000 ng mL−1 for OH-ITZ, it was very accurate and it gave very good inter- and intra-day precisions. The proposed high-throughput method was employed in a bioequivalence study after per os administration of two 100 mg tablets of ITZ, and it allowed this study to be completed in under four days.



Similar content being viewed by others
References
Wong JW, Nisar Ur-Rand, Yuen KH (2003) J Chromatogr B 798:355–360
Srivatsan V, Dasgupta AK, Kale P, Datla RR, Soni D, Patel M, Patel R, Mavadhiya C (2004) J Chromatogr A 1031:307–313
Gubbins PO, Gurley BJ, Bowman J (1998) J Pharm Biomed Anal 16:1005–1012.
Compas D, Touw DJ, De Goede PNFC (1996) J Chromatogr B 687:453–456
Zhong L, Eisenhandler R, Yeh KC (2001) J Mass Spectrom 36:736–741
Chen X, Zhong D, Liu D, Wang Y, Han Y, Gu J (2003) Rapid Commun Mass Spectrom 17:2459–2463
Dotsikas Y, Kousoulos C, Tsatsou G, Loukas YL (2005) Rapid Commun Mass Spectrom 19:2055–2061
Carrier A, Parent J (2000) J Chromatogr B 745:413–420
Yao M, Chen L, Srinivas LR (2001) J Chromatogr B 752:9–16
Vogeser M, Spöhrer U, Schiel X (2003) Chem Lab Med 41:915–920
Jemal M (2000) Biomed Chromatogr 14:422–429
Basileo G, Breda M, Fonte G, Pisano R, James CA (2003) J Pharm Biomed Anal 32:591–600
Kitchen CJ, Wang AQ, Musson DG, Yang AY, Fisher AL (2003) J Pharm Biomed Anal 31:647–654
Zhang N, Yang A, Rogers JD, Zhao JJ (2004) J Pharm Biomed Anal 34:175–187
Barattè S, Sarati S, Frigerio E, James CA, Ye C, Zhang Q (2004) J Chromatogr A 1024:87–94
Zhang J, Zeng W, Kitchen C, Wang AQ, Musson DG (2004) J Chromatogr B 806:167–175
Zhang N, Hoffman KL, Li W, Rossi DT (2000) J Pharm Biomed Anal 22:131–138
Xue YJ, Pursley J, Arnold ME (2004) J Pharm Biomed Anal 34:369–378
CDER (2001) Guidance for industry: Bioanalytical method validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Rockville, MD
Bonfiglio R, King RC, Olah TV, Merkle K (1999) Rapid Commun Mass Spectrom 13:1175–1185
Annesley TM (2003) Clin Chem 49:1041–1044
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kousoulos, C., Tsatsou, G., Apostolou, C. et al. Development of a high-throughput method for the determination of itraconazole and its hydroxy metabolite in human plasma, employing automated liquid–liquid extraction based on 96-well format plates and LC/MS/MS. Anal Bioanal Chem 384, 199–207 (2006). https://doi.org/10.1007/s00216-005-0159-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-0159-6