Abstract
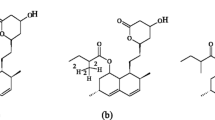
The aim of the present study was to develop a chromatographic method for the analysis of atorvastatin, o- and p-hydroxyatorvastatin (acid and lactone forms) in human plasma after administration of atorvastatin at the lowest registered dose (10 mg) in clinical studies. Sample preparation was performed by solid-phase extraction and was followed by separation of the analytes on an HPLC system with a linear gradient and a mobile phase consisting of acetonitrile, water and formic acid. Detection was achieved by tandem mass spectrometry operated in the electrospray positive ion mode. Validation of the method for the compounds for which reference compounds were available (acid forms of atorvastatin, o- and p-hydroxyatorvastatin) showed linearity within the concentration range (0.2–30 ng/ml for atorvastatin acid and p-hydroxyatorvastatin acid, and 0.5–30 ng/ml for o-hydroxyatorvastatin acid) (r2≥0.99, n=5 for all analytes). Accuracy and precision (evaluated at 0.5, 3 and 30 ng/ml for atorvastatin, p-hydroxyatorvastatin and 1, 3 and 30 ng/ml for o-hydroxyatorvastatin) were both satisfactory. The detection limit was 0.06 ng/ml for atorvastatin and p-hydroxyatorvastatin, and 0.15 ng/ml for o-hydroxyatorvastatin. The method has been successfully applied in a clinical study where atorvastatin, o- and p-hydroxyatorvastatin (both acid and lactone forms) could be detected in a 24-h sampling interval after administration of the lowest registered dose of atorvastatin (10 mg) for one week.




Similar content being viewed by others
References
Kearney A, Crawford L, Mehta S, Radebaugh G (1993) Pharm Res 10:1461–1465
Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH, Pearson PG, Baillie TA (2002) Drug Metab Dispos 30:505–512
Kantola T, Kivisto K, Neuvonen P (1998) Clin Pharmacol Ther 64:58–65
Parke-Davis (2004) Product information: Lipitor (Atorvastatin Calcium). Parke-Davis (Division of Pfizer Inc.), Ann Arbor, MI
Asberg A, Hartmann A, Fjeldså E, Bergan S, Holdaas H (2001) Am J Transplant 1:382–386
Amsden GW, Kuye O, Wei GC (2002) J Clin Pharmacol 42:444–449
Hsyu PH, Schultz-Smith MD, Lillibridge JH, Lewis RH, Kerr BM (2001) Antimicrob Agents Chemother 45:3445–3450
Siedlik PH, Olson SC, Yang BB, Stern RH (1999) J Clin Pharmacol 39:501–504
Bullen W, Miller R, Hayes R (1999) J Am Soc Mass Spectrom 10:55–66
Jemal M, Ouyang Z, Chen BC, Teitz D (1999) Rapid Commun Mass Sp 13:1003–1015
Lins RL, Matthys KE, Verpooten GA, Peeters PC, Dratwa M, Stolear JC, Lameire NH (2003) Nephrol Dial Transplant 18:967–976
Jemal M, Xia YQ (2000) J Pharm Biomed Anal 22:813–827
Hermann M, Asberg A, Christensen H, Holdaas H, Hartmann A, Reubsaet JL (2004) Clin Pharmacol Ther 76:388–391
Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A (2000) Pharm Res 17:1551–1557
Molden E, Helen Boe G, Christensen H, Reubsaet L (2003) J Pharm Biomed Anal 33:275–285
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hermann, M., Christensen, H. & Reubsaet, J.L.E. Determination of atorvastatin and metabolites in human plasma with solid-phase extraction followed by LC–tandem MS. Anal Bioanal Chem 382, 1242–1249 (2005). https://doi.org/10.1007/s00216-005-3266-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-3266-5