Abstract
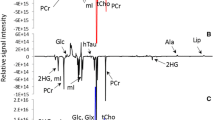
Fourier transform infrared (FT-IR) spectroscopic imaging has been used to characterize different types of pituitary gland tumors and normal pituitary tissue. Freshly resected tumor tissue from surgery was prepared as thin cryosections and examined by FT-IR spectroscopic imaging. Tissue types were discriminated via k-means cluster analysis and a supervised classification algorithm based on linear discriminant analysis. Spectral classification allowed us to discriminate between tumor and non-tumor cells, as well as between tumor cells that produce human growth hormone (hGH+) and tumor cells that do not produce that hormone (hGH−).The spectral classification was compared and contrasted with a histological PAS and orange G stained image. It was further shown that hGH+ pituitary tumor cells show stronger amide bands than tumor cells that do not produce hGH. This study demonstrates that FT-IR spectroscopic imaging can not only potentially serve as a fast and objective approach for discriminating pituitary gland tumors from normal tissue, but that it can also detect hGH-producing tumor cells.








Similar content being viewed by others
References
Hornyak M, Digre K, Couldwell WT (2009) Neuro-ophthalmologic manifestations of benign anterior skull base lesions. Postgrad Med 121(4):103–114
Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S (2007) Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 15(2):203–216
Vandeva S, Jaffrain-Rea ML, Daly AF, Tichomirowa M, Zacharieva S, Beckers A (2010) The genetics of pituitary adenomas. Best Practice Res Clinical Endocrin Matabol 24(9):461–476
Kreutzer J, Fahlbusch R (2004) Diagnosis and treatment of pituitary tumors. Curr Opin Neurol 17(6):693–703
Al-Brahim NYY, Asa SL (2006) My approach to pathology of the pituitary gland. J Clin Pathol 59:1245–1253
Saraswathy A, Jayasree RS, Baiju KV, Gupta AK, Pillai VPM (2009) Optimum wavelength for the differentiation of brain tumor tissue using autofluorescence spectroscopy. Photomed Laser Surg 27(3):425–433
Everlin LS, Dill AL, Golby AJ, Ligon KL, Wiseman JM, Cooks RG, Agar NYR (2010) Discrimination of human astrocytoma subtypes by lipid snalysis using desorption electrospray ionization imaging mass spectrometry. Angew Chem Int Ed 49:5993–5956
Lee LS, Lin SY, Chi CW, Liu HC, Cheng CL (1995) Non-destructive analysis of the protein conformational structure of human pituitary adenomas using reflectance FT-IR microspectroscopy. Cancer Lett 94:65–69
Carmona P, Molina M, Lasagabaster (1994) Structural study of human growth hormone-releasing factor fragment (1-29) by vibrational spectroscopy. Spectrochim Acta 51A(5):929–938
Steiner G, Küchler S, Hermann A, Koch E, Salzer R, Schackert G, Kirsch M (2008) Rapid and label-free classification of human glioma cells by infrared spectroscopic imaging. Cytometry 73A:1158–1164
Krafft C, Shapoval L, Sobottka SB, Geiger K, Schackert G, Salzer R (2006) Identification of primary tumors of brain metastases by SIMCA classification of IR spectroscopic images. Biochim Biophys Acta Biomembranes 1758:883–891
Kirsch M, Schackert G, Salzer R, Krafft C (2010) Raman spectroscopic imaging for in vivo detection of cerebral brain metastases. Anal Bioanal Chem 398:1707–1713
Mackanos MA, Contag CH (2010) Fiber-optic probes enable cancer detection with FTIR spectroscopy. Trends Biotechnol 28:317–323
Stelling A, Salzer R, Kirsch M, Sobottka SB, Geiger K, Koch E, Schackert G, Steiner G (2011) Intra-operative optical diagnostics with vibrational spectroscopy. Anal Bioanal Chem 400:2745–2753
Liu Y, Xu Y, Liu Y, Zhang Y, Wang D, Xiu D, Xu Z, Zhou X, Wu J, Ling X (2011) Detection of cervical metastatic lymph nodes in papillary thyroid carcinoma by Fourier transform infrared spectroscopy. Br J Surg 98:380–394
Rehman S, Movasaghi Z, Darr JA, Rehman IU (2010) Fourier transform infrared spectroscopic analysis of breast cancer tissues; identifying differences between normal breast, invasive ductal carcinoma, and ductal carcinoma in situ of the breast. Appl Spectr Rev 45:355–368
Salzer R, Siesler HW (2009) Infrared and Raman spectroscopic imaging. Wiley–VCH, Weinheim
Mordechai S, Sahu RK, Hammody Z, Mark S, Kantarovich K, Guterman H, Podshyvalov A, Goldstein J, Argov S (2004) Possible common biomarkers from FTIR microspectroscopy of cervical cancer and melanoma. J Microscopy 215:86–91
Krafft C, Steiner G, Beleites C, Salzer R (2009) Disease recognition by infrared and Raman spectroscopy. J Biophotonics 2(1–2):13–28
Beleites C, Baumgartner R, Bowmann C, Somorjai R, Steiner G, Salzer R, Sowa MG (2005) Variance reduction in estimating classification error using sparse dataset. Chemom Intell Lab Syst 79:91–100
Halmi NS (1978) Immunostaining of growth hormone and prolactin in paraffin-embedded and stored or previously stained materials. J Histochem Cytochem 26(7):486–495
Tibshirani R, Walther G, Hastie T (2001) Estimating the number of clusters in a dataset via the gap statistic. J R Statist Soc B 63:411–423
Krafft C, Sobottka SB, Geiger KD, Schackert G, Salzer R (2007) Classification of malignant gliomas by infrared spectroscopic imaging and linear discriminant analysis. Anal Bioanal Chem 387(5):1669–1677
Steiner G, Shaw A, Choo-Smith LP, Abuid MH, Schackert G, Sobottka S, Steller W, Salzer R, Mantsch HH (2003) Distinguishing and grading human gliomas by IR spectroscopy. Biopolymers 72(6):464–471
Zhan X, Mesiderio DM (2005) Comparative proteomics analysis of human pituitary adenomas: current status and future perspectives. Mass Spectr Rev 24:783–813
Mulinacci F, Bell SEJ, Capella MAH, Gurny R, Arvinte T (2011) Oxidized recombinant human growth hormone that maintains conformational integrity. J Pharm Sci 100(1):110–122
Lloyd RV, Jin L, Chandler WF, Horvath E, Stefaneanu L, Kovacs K (1993) Pituitary specific transcription factor messenger ribonucleic expression in adenomatous and nontumorous human pituitary tissues. Lab Invest 69(5):570–575
Otsuka F, Tamiya T, Yamauchi T, Ogura T, Ohmoto T, Makino H (1999) Quantitative analysis of growth-related factors in human pituitary adenomas. Lowered insulin-like growth factor-I and its receptor mRNA in growth hormone-producing adenomas. Regul Pept 83(1):31–38
Oka H, Kameya T, Sato Y, Naritaka H, Kawano N (1999) Significance of growth hormone-releasing hormone receptor mRNA in non-neoplastic pituitary and pituitary adenomas: a study by RT-PCR and in situ hybridization. J Neurooncol 41(3):197–204
Fujioka N, Morimoto Y, Arai T, Kikuchi M (2004) Discrimination between normal and malignant human gastric tissue by Fourier transform infrared spectroscopy. Cancer Detection Prevent 28:32–36
Fabian H, Jackson M, Murphy L, Watson PH, Fichtner I, Mantsch HH (1995) A comparative infrared spectroscopic study of human breast tumors and breast tumor cell xenografts. Biospectr 1:37–45
Eljamel MS (2009) Which intracranial lesions would be suitable for 5-aminolevulenic acid-induced fluorescence-guided identification, localization, or resection? A prospective study of 114 consecutive intracranial lesions. J Neuro Onc 56:93–97
Acknowledgments
This work was supported by the Federal Ministry of Education and Research, Germany (no. 13N10777). The authors thank Elke Leipnitz for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Professor Reiner Salzer on the occasion of his 70th birthday to honor his substantial achievements in analytical chemistry and vibrational spectroscopy.
Rights and permissions
About this article
Cite this article
Steiner, G., Mackenroth, L., Geiger, K.D. et al. Label-free differentiation of human pituitary adenomas by FT-IR spectroscopic imaging. Anal Bioanal Chem 403, 727–735 (2012). https://doi.org/10.1007/s00216-012-5824-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-5824-y