Abstract
This paper describes a novel analytical methodology for the simultaneous determination of absolute and total concentrations of 11 native thyroid hormones and associated metabolites, viz. thyroxine (T4), 3,3′, 5-triiodothyronine (T3), 3,3′, 5′-triiodothyronine (rT3), 3,5-diiodothyronine (3,5-T2), 3,3′- diiodothyronine (3,3′-T2), 3-iodothyronine (T1), thyronine (T0), 3-iodothyronamine (T1AM), tetraiodothyroacetic acid (Tetrac), triiodothyroacetic acid (Triac), and diiodothyroacetic acid (Diac), in 50-μL of plasma or serum. The method was optimized using four isotopic labeled surrogate and internal standards in combination with solid-phase extraction and LC-MS/MS. The methodology was further evaluated using amphibian plasma and serum with matrix-matched calibration applied for quantification. Method detection limits are 3.5 pg T4, 1.5 pg T3, 2.9 pg rT3, 1.7 pg 3,3′–T2, 2.3 pg 3,5-T2, and between 0.3 and 7.5 pg for the remaining six metabolites in 50 μL aliquots of blood sera or plasma. Accuracies and repeatabilities for all analytes were between 88 and 103 % and 1.31 and 17.2 %, respectively. Finally, we applied the method on adult frog (Xenopus laevis) plasma and tadpole (Rana (Lithobates) catesbeiana) serum. We observed up to seven different thyroid hormones and associated metabolites in tadpole serum. This method will enable researchers to improve the assessment of thyroid homeostasis and endocrine disruption in animals and humans.

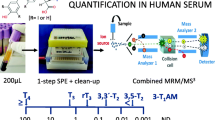
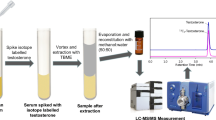
Quantification of 11 thyroid hormones and metabolites from 50 μL plasma or serum using protein denaturation in combination with solid-phase extraction followed by LC-MS/MS.






Similar content being viewed by others
References
Kendall EC. Thyroxine. New York: Chemical Catalog Company, Inc; 1929.
Senese R, Cioffi F, de Lange P, Goglia F, Lanni A. Thyroid: biological actions of “nonclassical” thyroid hormones. J Endocrinol. 2014;221:R1–12.
De Groot LJ, editors. Endocrinology Adult and Pediatric: The Thyroid Gland. 6th ed. Elsevier Health Sciences; 2013.
Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122:3035–43.
Braverman LE, Cooper DS, editors. Werner and Ingbar's The Thyroid. 10th ed. Lippincott: Williams and Wilkins.
Harvey CB, Williams GR. Mechanism of thyroid hormone action. Thyroid. 2002;12:441–6.
Piehl S, Hoefig CS, Scanlan TS, Köhrle J. Thyronamines—Past, Present, and Future. Endocr Rev. 2011;32:64–80.
Noyes PD, Lema SC, Roberts SC, Cooper EM, Stapleton HM. Rapid method for the measurement of circulating thyroid hormones in low volumes of teleost fish plasma by LC-ESI/MS/MS. Anal Bioanal Chem. 2014;406:715–26.
Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, Lin ET, Hatton D, Zucchi R, Grandy DK. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10(6):638–642.
Kelly GS. Peripheral metabolism of thyroid hormones: a review. Altern Med Rev. 2000;5:306–333.
U.S. NLM, ChemIDplus. United State National Library of Medicine; 2003.
Holm SS, Hansen SH, Faber J, Staun-Olsen P. Reference methods for the measurement of free thyroid hormones in blood. Clin Biochem. 2004;37:85–93.
Carvalho VM. The coming of age of liquid chromatography coupled to tandem mass spectrometry in the endocrinology laboratory. J of Chromatogr B 2012;883-884:50–58.
Soldin OP, Soldin SJ. Thyroid hormone testing by tandem mass spectrometry. Clin Biochem. 2011;44:89–94.
Wu AHB, French D. Implementation of liquid chromatography/mass spectrometry into the clinical laboratory. Clin Chim Acta 2013;420:4–10.
Wang D, Stapleton HM. Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2010;397:1831–9.
Kunisue T, Fisher JW, Kannan K. Modulation of thyroid hormone concentrations in serum of rats co-administered with perchlorate and iodide-deficient diet. Arch Environ Contam Toxicol. 2011;61:151–8.
Taylor AC, Kollros JJ. Stages in the normal development of Rana pipiens larvae. Anat Rec. 1946;94:7–23.
Food and Drug Administration. Validation of Analytical Methods VICH GL49(R); 2015.
Vial J, Jardy A. Experimental comparison of the different approaches to estimate LOD and LOQ of an HPLC method. Anal Chem. 1999;71:2672–7.
Hopley CJ, Stokes P, Webb KS, Baynham M. The analysis of thyroxine in human serum by an “exact matching” isotope dilution method with liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:1033–8.
De Brabandere VI, Hou P, Stöckl D, Thienpont LM, De Leenheer AP. Isotope dilution-liquid chromatography/electrospray ionization-tandem mass spectrometry for the determination of serum thyroxine as a potential reference method. Rapid Commun Mass Spectrom. 1998;12:1099–103.
Luna LG, Coady K, McFadden JR, Markham DA, Bartels MJ. Quantification of Total Thyroxine in Plasma from Xenopus laevis. J Anal Toxicol. 2013;37:326–36.
Kunisue T, Eguchi A, Iwata H, Tanabe S, Kannan K. Analysis of thyroid hormones in serum of Baikal seals and humans by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and immunoassay methods: application of the LC-MS/MS method to wildlife tissues. Environ Sci Technol. 2011;45:10140–7.
Ackermans MT, Klieverik LP, Ringeling P, Endert E, Kalsbeek A, Fliers E. An online solid-phase extraction-liquid chromatography-tandem mass spectrometry method to study the presence of thyronamines in plasma and tissue and their putative conversion from 13C6-thyroxine. J Endocrinol. 2010;206:327–34.
Hackenmueller SA, Scanlan TS. Identification and quantification of 3-iodothyronamine metabolites in mouse serum using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1256:89–97.
Gu J, Soldin OP, Soldin SJ. Simultaneous quantification of free triiodothyronine and free thyroxine by isotope dilution tandem mass spectrometry. Clin Biochem. 2007;40:1386–1391.
Soldin SJ, Soukhova N, Janicic N, Jonklaas J, Soldin OP. The measurement of free thyroxine by isotope dilution tandem mass spectrometry. Clin Chim Acta. 2005;358:113–8.
Tai SS-C, Sniegoski LT, Welch MJ. Candidate reference method for total thyroxine in human serum use of isotope-dilution liquid chromatography-mass spectrometry with electrospray ionization. Clin Chem. 2002;48:637–42.
Tóth G, Hosztafi S, Kovács Z, Noszál B. The site-specific basicity of thyroid hormones and their precursors as regulators of their biological functions. J Pharm Biomed Anal. 2012;61:156–64.
Organization for Economic Cooperation and Development. Test no. 111: hydrolysis as a function of pH. Paris: OECD Publishing; 2004.
Svanfelt J, Eriksson J, Kronberg L. Photochemical transformation of the thyroid hormone levothyroxine in aqueous solution. Environ Sci Pollut Res. 2011;18:871–6.
van der Walt B, Cahnmann HJ. Synthesis of thyroid hormone metabolites by photolysis of thyroxine and thyroxine analogs in the near UV. Proc Natl Acad Sci USA. 1982;79:1492–1496.
Tai SS-C, Bunk DM, White E, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of total 3,3',5-triiodothyronine in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2004;76:5092–6.
Stahnke H, Kittlaus S, Kempe G, Alder L. Reduction of matrix effects in liquid chromatography-electrospray ionization-mass spectrometry by dilution of the sample extracts: how much dilution is needed? Anal Chem. 2012;84:1474–82.
Hansen M, Jacobsen NW, Nielsen FK, Björklund E, Styrishave B, Halling-Sørensen B. Determination of steroid hormones in blood by GC-MS/MS. Anal Bioanal Chem. 2011;400:3409–17.
Hansen M, Poulsen R, Luong X, Sedlak DL, Hayes T. Liquid chromatography tandem mass spectrometry method using solid-phase extraction and bead-beating-assisted matrix solid-phase dispersion to quantify the fungicide tebuconazole in controlled frog exposure study: analysis of water and animal tissue. Anal Bioanal Chem. 2014;406:7677–85.
Souverain S, Rudaz S, Veuthey JL. Protein precipitation for the analysis of a drug cocktail in plasma by LC-ESI-MS. J Pharm Biomed Anal. 2004;35:913–20.
Jonas W, Lietzow J, Wohlgemuth F, Hoefig CS, Wiedmer P, Schweizer U, Köhrle J, Schürmann A. 3,5-diiodo-L-thyronine (3,5-T2) exerts thyromimetic effects on hypothalamus-pituitary-thyroid axis, body composition, and energy metabolism in male diet-induced obese mice. Endocrinology. 2015;1:389–399.
Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin). A systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Amsterdam: North-Holland Publishing Company; 1956.
Acknowledgments
This work was financially supported by the European Commission Seventh Framework Program, grant no. PIOF-GA-2012-329996, and in part by the Engineering Research Center for Reinventing the Nation’s Water Infrastructure (ReNUWIt) EEC-1028968 at the University of California, Berkeley. T.H. and X.L. are supported by the Kapor Foundation, the Ceres Foundation, and Beyond Pesticides. C.C.H. is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). Dr. Anthony Iavarone at QB3 Chemistry Mass Spectrometry Facility, UC Berkeley and Professor Erland Björklund at Kristianstad University are gratefully acknowledged for fruitful discussions and contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Ethical standards
University of California and University of Victoria animal care and use programs, protocols, and procedures were followed and appropriate permits obtained.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 49 kb)
Rights and permissions
About this article
Cite this article
Hansen, M., Luong, X., Sedlak, D.L. et al. Quantification of 11 thyroid hormones and associated metabolites in blood using isotope-dilution liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 408, 5429–5442 (2016). https://doi.org/10.1007/s00216-016-9614-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9614-9