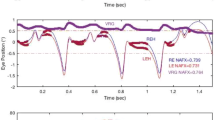
Abstract
We recently described an area in the ferret posterior suprasylvian (PSS) cortex characterized by a high proportion of direction selective neurons. To answer the question whether area PSS subserves functions similar to cat posteromediolateral suprasylvian area (PMLS) and monkey medial temporal area (MT) we investigated the contribution of area PSS to visual motion perception and optokinetic nystagmus. Ferrets were tested on global motion detection before and after bilateral lesions involving area PSS and control lesions of other extrastriate visual areas. Following PSS lesions motion coherence thresholds were significantly increased both in pigmented and albino ferrets, whereas control lesions sparing PSS did not affect visual motion perception. Optokinetic nystagmus was strongly reduced to absent after PSS lesions. These results indicate that area PSS is crucial for global motion processing in the ferret and in that sense may be functionally equivalent to PMLS in the cat and area MT in the monkey.










Similar content being viewed by others
References
Abadi RV, Pascal E (1994) Periodic alternating nystagmus in humans with albinism. Invest Ophthalmol Vis Sci 35:4080–4086
Albright TD, Desimone R, Gross CG (1984) Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophysiol 51:16–31
Baker GE, Thompson ID, Krug K, Smythe D, Tolhurst DJ (1998) Spatial-frequency tuning and geniculocortical projections in the visual cortex (areas 17 and 18) of the pigmented ferret. Eur J Neurosci 10:2657–2668
Beardsley SA, Vaina LM (2006) Global motion mechanisms compensate local motion deficits in a patient with a bilateral occipital lobe lesion. Exp Brain Res 173:724–732
Berson DM, Graybiel AM (1980) Some cortical and subcortical fiber projections to the accessory optic nuclei in the cat. Neuroscience 5:2203–2217
Blanke O, Landis T, Mermoud C, Spinelli L, Safran AB (2003) Direction-selective motion blindness after unilateral posterior brain damage. Eur J Neurosci 18:709–722
Brosseau-Lachaine O, Faubert J, Casanova C (2001) Functional sub-regions for optic flow processing in the posteromedial lateral suprasylvian cortex of the cat. Cereb Cortex 11:989–1001
Cantone G, Xiao J, Levitt JB (2006) Retinotopic organization of ferret suprasylvian cortex. Vis Neurosci 23:61–77
Collewijn H, Winterson BJ, Dubois MF (1978) Optokinetic eye movements in albino rabbits: inversion in anterior visual field. Science 199:1351–1353
Collewijn H, Apkarian P, Spekreijse H (1985) The oculomotor behaviour of human albinos. Brain 108:1–18
Demer JL, Zee DS (1984) Vestibulo-ocular and optokinetic deficits in albinos with congenital nystagmus. Invest Ophthalmol Vis Sci 25:739–745
Distler C, Mustari M, Hoffmann K-P (2002) Cortical projections to the nucleus of the optic tract and dorsal terminal nucleus and to the dorsolateral pontine nucleus in macaques: a dual retrograde tracing study. J Comp Neurol 444:144–158
Distler C, Korbmacher H, Hoffmann K-P (2006) Neuronal connections of motion sensitive area PSS of the ferret (Mustela putorius furo). FENS Forum Abstracts 3:A216.4
Duersteler MR, Wurtz RH (1988) Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol 60:940–965
Fox JG (1998) Biology and diseases of the ferret. Lippincott Williams and Wilkins, Philadelphia
Gallyas F (1979) Silver staining of myelin by means of physical development. Neurol Res 1:203–209
Guo K, Benson PJ, Blakemore C (1998) Residual motion discrimination using colour information without primary visual cortex. Neuroreport 9:2103–2107
Hahnenberger RW (1977) Differences in optokinetic nystagmus between albino and wildtype rabbits. Exp Eye Res 25:9–17
Hein A, Courjon JH, Flandrin JM, Arzi M (1990) Optokinetic nystagmus in the ferret: including selected comparisons with the cat. Exp Brain Res 79:623–632
Hess DT, Merker BH (1983) Technical modifications of Gallyas’ silver stain for myelin. J Neurosci Methods 8:95–97
Hoffmann K-P, Garipis N, Distler C (2004) Optokinetic deficits in albino ferrets (Mustela putorius furo): a behavioural and electrophysiological study. J Neurosci 24:4061–4069
Hupfeld D, Distler C, Hoffmann K-P (2006) Motion perception deficits in albino ferrets (Mustela putorius furo). Vision Res 46:2941–2948. doi 10.1016/j.visres.2006.02.020
Huxlin KR, Pasternak T (2004) Training-induced recovery of visual motion perception after extrastriate cortical damage in the adult cat. Cereb Cortex 14:81–90
Innocenti GM, Manger PR, Masiello I, Colin I, Tettoni L (2002) Architecture and callosal connections of visual areas 17, 18, 19 and 21 in the ferret (Mustela putorius). Cereb Cortex 12:411–422
Jeffery G (1997) The albino retina: an abnormality that provides insight into normal retinal development. Trends Neurosci 20:165–169
Kawamura S, Sprague JM, Niimi K (1974) Corticofugal projections from the visual cortices to the thalamus, pretectum and superior colliculus in the cat. J Comp Neurol 158:339–362
Komatsu H, Wurtz RH (1988) Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol 60:580–603
Lagae L, Raiguel S, Orban GA (1993) Speed and direction selectivity of macaque middle temporal neurons. J Neurophysiol 69:19–39
Lauwers K, Saunders R, Vogels R, Vandenbussche E, Orban GA (2000) Impairment in motion discrimination tasks is unrelated to amount of damage to superior temporal sulcus motion areas. J Comp Neurol 420:539–557
Li B, Li BW, Chen Y, Wang LH, Diao YC (2000) Response properties of PMLS and PLLS neurons to simulated optic flow patterns. Eur J Neurosci 12:1534–1544
Lomber SG, Cornwell P, Sun JS, MacNeil MA, Payne BR (1994) Reversible inactivation of visual processing operations in middle suprasylvian cortex of the behaving cat. Proc Natl Acad Sci USA 91:2999–3003
Lynch JC, McLaren JW (1983) Optokinetic nystagmus deficits following parieto-occipital cortex lesions in monkeys. Exp Brain Res 49:125–130
Manger PR, Kiper D, Masiello I, Murillo L, Tettoni L, Hunyadi Z, Innocenti GM (2002) The representation of the visual field in three extrastriate areas of the ferret (Mustela putorius) and the relationship of retinotopy and field boundaries to callosal connectivity. Cereb Cortex 12:423–437
Manger PR, Masiello I, Innocenti GM (2002) Areal organization of the posterior parietal cortex of the ferret (Mustela putorius). Cereb Cortex 12:1280–1297
Manger PR, Nakamura1 H, Valentiniene S, Innocenti GM (2004) Visual areas in the lateral temporal cortex of the ferret (Mustela putorius). Cereb Cortex 14:676–689
Marcotte RR, Updyke BV (1982) Cortical visual areas of the cat project differentially onto the nuclei of the accessory optic system. Brain Res 242:205–217
Maunsell JH, Van Essen DC (1983) Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol 49:1127–1147
Maunsell JH, Van Essen DC (1983) The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci 3:2563–2586
Moore BD, Alitto HJ, Usrey WM (2005) Orientation tuning, but not direction selectivity, is invariant to temporal frequency in primary visual cortex. J Neurophysiol 94:1336–1345
Nakamura H, Kashii S, Nagamine T, Matsui Y, Hashimoto T, Honda Y, Shibasaki H (2003) Human V5 demonstrated by magnetoencephalography using random dot kinematograms of different coherence levels. Neurosci Res 46:423–433
Newsome WT, Paré EB (1988) A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J Neurosci 8:2201–2211
Newsome WT, Wurtz RH, Duersteler MR, Mikami A (1985) Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci 5:825–840
Nguyen AP, Spetch ML, Crowder NA, Winship IR, Hurd PL, Jantzie LL, Wylie DR (2004) A dissociation of motion and spatial-pattern vision in the avian telencephalon: implications for the evolution of “visual streams”. J Neurosci 24:4962–4970
Palmer SM, Rosa MGP (2006) A distinct anatomical network of cortical areas for analysis of motion in far peripheral vision. Eur J Neurosci 24:2389–2405
Pasternak T, Merigan WH (1994) Motion perception following lesions of the superior temporal sulcus in the monkey. Cereb Cortex 4:247–259
Pasternak T, Tompkins J, Olson CR (1995) The role of striate cortex in visual function of the cat. J Neurosci 15:1940–1950
Payne BR (1993) Evidence for visual cortical area homologs in cat and macaque monkey. Cereb Cortex 3:1–25
Philipp R, Distler C, Hoffmann K-P (2006) A motion-sensitive area in ferret extrastriate visual cortex: an analysis in wildtype and albino animals. Cereb Cortex 16:779–790
Rauschecker JP, von Grunau MW, Poulin C (1987) Centrifugal organization of direction preferences in the cat’s lateral suprasylvian visual cortex and its relation to flow field processing. J Neurosci 7:943–958
Rudolph KK, Pasternak T (1996) Lesions in cat lateral suprasylvian cortex affect the perception of complex motion. Cereb Cortex 6:814–822
Rudolph KK, Pasternak T (1999) Transient and permanent deficits in motion perception after lesions of cortical areas MT and MST in the macaque monkey. Cereb Cortex 9:90–100
Schenk T, Zihl J (1997) Visual motion perception after brain damage: I. Deficits in global motion perception. Neuropsychologia 35:1289–1297
Schmidt KE, Castelo-Branco M, Goebel R, Payne BR, Lomber SG, Galuske RAW (2006) Pattern motion selectivity in population responses of area 18. Eur J Neurosci 24:2363–2374
Sherk H (1988) Retinotopic order and functional organization in a region of suprasylvian visual cortex, the Clare-Bishop area. In: Hicks TP, Benedek G (eds) Progress in brain research, vol. 75: vision within extrageniculostriate systems, Elsevier, Amsterdam, pp 237–244
Sherk H, Fowler GA (2002) Lesions of extrastriate cortex and consequences for visual guidance during locomotion. Exp Brain Res 144:159–171
Spear PD, Baumann TP (1975) Receptive field characteristics of single neurons in lateral suprasylvian visual area of the cat. J Neurophysiol 38:1403–1420
Spear PD, Miller S, Ohman L (1983) Effects of lateral suprasylvian visual cortex lesions on visual localization, discrimination, and attention in cats. Behav Brain Res 10:339–359
Standage GP, Benevento LA (1983) The organization of connections between the pulvinar and visual area MT in the macaque monkey. Brain Res 262:288–294
St John RS, Fisk JD, Timney B, Goodale MA (1984) Eye movements of human albinos. Am J Optom Physiol Opt 61:377–385
Strong NP, Malach R, Lee P, Van Sluyters RC (1984) Horizontal optokinetic nystagmus in the cat: recovery from cortical lesions. Brain Res 315:179–92
Toyama K, Mizobe K, Akase E, Kaihara T (1994) Neuronal responsiveness in areas 19 and 21a, and the posteromedial lateral suprasylvian cortex of the cat. Exp Brain Res 99:289–301
Tusa RJ, Demer JL, Herdman SJ (1989) Cortical areas involved in OKN and VOR in cats: cortical lesions. J Neurosci 9:1163–1178
Ungerleider LG, Desimone R, Galkin TW, Mishkin M (1984) Subcortical projections of area MT in macaques. J Comp Neurol 223:368–386
Vaina LM, Soloviev S, Bienfang DC, Cowey A (2000) A lesion of cortical area V2 selectively impairs the perception of the direction of first-order visual motion. Neuroreport 11:1039–1044
Vaina LM, Cowey A, Jakab M, Kikinis R (2005) Deficits of motion integration and segregation in patients with unilateral extrastriate lesions. Brain 128:2134–2145
Ventre J (1985) Cortical control of oculomotor functions. I. Optokinetic nystagmus. Behav Brain Res 15:211–226
von Bonin G, Bailey P (1947) The neocortex of macaca mulatta. University of Illinois Press, Urbana
Zeki S (1991) Cerebral akinetopsia (visual motion blindness). A review. Brain 114:811–824
Acknowledgements
We thank B. Krekelberg for creating the stimulus software “randomdots” and S. Krämer, H. Korbmacher, and S. Dobers for expert technical assistance. We are also grateful for the constructive comments of the anonymous reviewers which helped to improve earlier versions of the manuscript. This study was supported by DFG grant Sonderforschungsbereich 509.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hupfeld, D., Distler, C. & Hoffmann, KP. Deficits of visual motion perception and optokinetic nystagmus after posterior suprasylvian lesions in the ferret (Mustela putorius furo). Exp Brain Res 182, 509–523 (2007). https://doi.org/10.1007/s00221-007-1009-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-1009-x