Abstract
Purpose
Several data mining algorithms (DMAs) are being studied in hopes of enhancing screening of large post-marketing safety databases for signals of novel adverse events (AEs). The objective of this study was to apply two DMAs to the United States FDA Adverse Event Reporting System (AERS) database to see whether signals of potentially fatal AEs with cancer drugs might have been identified earlier than with traditional methods.
Methods
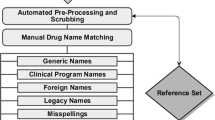
Screening algorithms used for analysis were the multi-item gamma Poisson shrinker (MGPS) and proportional reporting ratios (PRRs). Data mining was performed on data from the FDA AERS database. When a signal was identified, it was compared with that in the year in which the event was added to package insert and/or the year a “case series” was published. A recent publication summarizing the time of dissemination of information on potentially fatal AEs to cancer drugs provided the data set for analysis.
Results
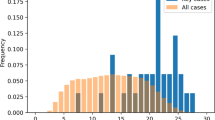
The peer-reviewed published analysis contained 21 drugs and 26 drug–event combinations (DECs) that were considered sufficiently specific for data mining. Twenty-four of the DECs generated a signal of disproportionate reporting with PRRs (6 at 1 year and 16 from 2 years to 18 years prior to either a published “case series” or a package insert change) and 20 with MGPS (3 at 1 year and 11 from 2 years to 16 years prior to either a published “case series” or a package insert change). Two DECs did not signal with either DMA.
Conclusion
At least one commonly cited DMA generated a signal of disproportionate reporting for 24 of 26 DECs for selected cancer drugs. For 16 DECs, one could conclude that a signal was generated well in advance (≥2 years) of standard techniques in use with at least one DMA. DMAs might be useful in supplementing traditional surveillance strategies with oncology drugs and other drugs with similar features. (i.e., drugs that may be approved on an accelerated basis, are known to have serious toxicity, are administered to patients with substantial and complicated comorbid illness, are not available to the general medical community, and may have a high frequency of “off-label” use).
Similar content being viewed by others
References
DuMouchel W (1999) Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat 53(3):177–190
Evans SJW, Waller PC, Davis S (2001) Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 10(6):483–486
Follman M, Michel A (2003) Proportional reporting ratios for signal detection in the drug safety database of a pharmaceutical company. Pharmacoepidemiol Drug Saf 12(suppl 1):S171
Gould AL (2003) Practical pharmacovigilance analysis strategies. Pharmacoepidemiol Drug Saf 12(7):559–574
Hauben M (2003) A brief primer on automated signal detection. Ann Pharmacother 37(7/8):1117–1123
Kawabe E (2003) Comparison of methodologies for signal detection using Japanese spontaneous reports. Pharmacoepidemiol Drug Saf 12(suppl 1):S169
Kilgour-Christie J, Czarnecki A, Simmons V (2001) Reporting ratio calculations from a pharmaceutical company database. Pharmacoepidemiol Drug Saf 10:S159
Ladewski LA, Belknap SM, Nebeker JR, et al (2003) Dissemination of information of potentially fatal adverse drug reactions for cancer drugs from 2000 to 2002: first results from the Research on Adverse Drug Events and Reports Project. J Clin Oncol 21:3859–3866
Lindquist M, Stahl M, Bate A (2002) A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf 23(6):533–542
Roux E, Tubert-Bitter P, Thiessard F, Fourrier A, et al (2003) Evaluation of data mining methods in pharmacovigilance using simulated data sets. Pharmacoepidemiol Drug Saf 12(suppl 1):S183
Szarfman A., Machado SG, O’Neill RT (2002) Use of screening algorithms and computer systems to efficiently signal higher-than expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf 25(6):381–392
U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Adverse Events Reporting System (AERS). http://www.fda.gov/cder/aers/default.htm
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hauben, M., Reich, L. & Chung, S. Postmarketing surveillance of potentially fatal reactions to oncology drugs: potential utility of two signal-detection algorithms. Eur J Clin Pharmacol 60, 747–750 (2004). https://doi.org/10.1007/s00228-004-0834-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0834-0