Abstract
Objective
The aim of the present study was to investigate the pharmacokinetic profile of tramadol hydrochloride in neonates, born from mothers who underwent analgesia with tramadol for the relief of labour pain.
Methods
Intramuscular tramadol (100–250 mg) was administered to 22 mothers giving birth who requested pain relief. At the time of birth (1.5–6.0 h after last tramadol dose), maternal and umbilical blood samples were taken. Another venous blood sample was drawn from each neonate, and at the same time from its mother, at 1, 2, 3, 6 or 12 h post-partum, providing the data for a population pharmacokinetic evaluation of tramadol and its metabolite M1. Routine APGAR scores and a standard neurological and adaptive capacity test were considered for evaluation of the effect of tramadol on the neonates.
Results
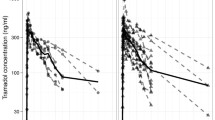
Serum tramadol concentrations at the time of birth (t0) were 243±102 ng/ml (mean±SD, umbilical vein), 258±103 ng/ml (umbilical artery) and 250±113 ng/ml (maternal vein). Serum M1 concentrations were 52±27 ng/ml (umbilical vein), 47±24 ng/ml (umbilical artery) and 56±21 ng/ml (maternal vein). The two-compartment type elimination profiles during the first 12 h post-partum for neonates (and mothers, respectively) were characterised by terminal t1/2 (tramadol)=7.0 (7.2) h and t1/2 (metabolite M1)=85.0 (5.5) h.
Conclusion
The intramuscular application of tramadol in birth-giving mothers almost freely reaches the neonate, confirming a high degree of placental permeability. The neonates already possess the complete hepatic capacity for the metabolism of tramadol into its active metabolite. However, the renal elimination of the active tramadol metabolite M1 is delayed, in line with the slow maturation process of renal function in neonates. Despite this difference in pharmacokinetics between neonates and adults, the intramuscular application of tramadol at the recommended dosage range during delivery appears to effective in the relief of labour pain.



Similar content being viewed by others
References
Chamberlain G, Wraight A, Steer P (1993) Pain and its relief in childbirth. The results of a national survey conducted by the National Birthday Trust. Churchill Livingstone, Edinburgh
Harmer M, Rosen M (1996) Parenteral opioids. In: Van Zundert A, Ostenheimer G (eds) Pain relief and anaesthesia in obstetrics. Churchill Livingstone, Edinburgh, pp 365–372
Bricker L, Lavender T (2002) Parenteral opioids for labor pain relief: a systematic review. Am J Obstet Gynecol 186:S94–S109
Lee CR, McTavish D, Sorkin EM (1993) Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states. Drugs 46:313–340
Lewis KS, Han NH (1997) Tramadol: a new centrally acting analgesic. Am J Health Syst Pharm 54:643–652
Vickers MD (1995) The efficacy of tramadol hydrochloride in the treatment of postoperative pain. Rev Contemp Pharmacother 6:499–506
Raffa RB, Nayak RK, Liao S, Minn FL (1995) The mechanism(s) of action and pharmacokinetics of tramadol hydrochloride. Rev Contemp Pharmacother 6:485–497
Cossmann M, Kohnen C (1995) General tolerability and adverse event profile of tramadol hydrochloride. Rev Contemp Pharmacother 6:513–531
McCartney C, Langford R (1995) The tolerability of tramadol hydrochloride in specific safety situations. Rev Contemp Pharmacother 6:533–539
Lintz W, Beier H, Gerloff J (1999) Bioavailability of tramadol after i.m. injection in comparison to i.v. infusion. Int J Clin Pharmacol Ther 37:175–183
Lintz W, Erlacin S, Frankus E, Uragg H (1981) Biotransformation of tramadol in man and animal. Arzneimittelforschung 31:1932–1943
Hennies HH, Friderichs E, Schneider J (1988) Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung 38:877–880
Bitsch M, Emmrich J, Hary J, Lippach G, Rindt W (1980) Obstetrical analgesia with tramadol. Fortschr Med 98:632–634
Prasertsawat PP, Herbabutya Y, Chaturachinda K (1986) Obstetric analgesia: comparison between tramadol, morphine and pethidine. Curr Ther Res Clin Exp 40:1022–1028
Husslein P, Kubista E, Egarter C (1987) Obstetrical analgesia with tramadol—results of a prospective randomized comparative study with pethidine. Z Geburtshilfe Perinatol 191:234–237
Kainz C, Joura E, Obwegeser R, Plockinger B, Gruber W (1992) Effectiveness and tolerance of tramadol with or without an antiemetic and pethidine in obstetric analgesia. Z Geburtshilfe Perinatol 196:78–82
Viegas OA, Khaw B, Ratnam SS (1993) Tramadol in labour pain in primiparous patients. A prospective comparative clinical trial. Eur J Obstet Gynecol Reprod Biol 49:131–135
Amiel-Tison C, Barrier G, Shnider SM, Levinson G, Hughes SC, Stefani SJ (1982) A new neurologic and adaptive capacity scoring system for evaluating obstetric medications in full-term newborns. Anesthesiology 56:340–350
Brockhurst NJ, Littleford JA, Halpern SH (2000) The neurologic and adaptive capacity score: a systematic review of its use in obstetric anesthesia research. Anesthesiology 92:237–246
Murthy BV, Pandy KS, Booker PD, Murray A, Lintz W, Terlinden R (2000) Pharmacokinetics of tramadol in children after i.v. or caudal epidural administration. Br J Anaesth 84:346–349
Le Guellec C, Autret-Leca E, Odoul F, Jonville Bera AP, Paintaud G (2001) Pharmacokinetic studies in neonatology: regulatory and methodologic problems. Therapie 56:663–668
Mirkin BL (1973) Drug disposition in pregnancy. In: Boreus L (ed) Fetal pharmacology. Raven Press, New York
Becker R, Lintz W (1986) Determination of tramadol in human serum by capillary gas chromatography with nitrogen-selective detection. J Chromatogr 377:213–220
Poulsen L, Arendt-Nielsen L, Brosen K, Sindrup SH (1996) The hypoalgesic effect of tramadol in relation to CYP2D6. Clin Pharmacol Ther 60:636–644
Ward RM, Mirkin BL (1994) Perinatal/Neonatal Pharmacology. In: Brody TM, Larner J, Minneman KP, Neu HC (eds) Human pharmacology: molecular to clinical. Mosby-Year Book Inc., St. Louis, pp 843–854
Suggs DM (2000) Pharmacokinetics in children: history, considerations, and applications. J Am Acad Nurse Pract 12:236–239
Hubner ME, Juarez ME (2002) The Apgar score. Is it still valid after a half century? Rev Med Chil 130:925–930
Acknowledgements
The authors are thankful to the mothers and neonates who participated in the study at the Nijmegen Hospitals. The study was sponsored by the Medical Department of Grünenthal GmbH, Aachen, Germany, and by ALTANA Pharma bv, Zwanenburg, the Netherlands. CobraMed AG—Institut für Klinische Arzneimittelstudien, Kerpen, Germany, performed the evaluation of the study. The authors declare that the experiments are compliant with the current laws of the country in which the experiment was performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Claahsen-van der Grinten, H.L., Verbruggen, I., van den Berg, P.P. et al. Different pharmacokinetics of tramadol in mothers treated for labour pain and in their neonates. Eur J Clin Pharmacol 61, 523–529 (2005). https://doi.org/10.1007/s00228-005-0955-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0955-0