Abstract
Objective
The 1995–2005 balance of EMEA activities in the field of paediatric medicines was evaluated, taking into account the number both of drugs authorised for children and paediatric studies supporting the Marketing Authorisation (MA).
Methods
Data on drugs authorised by EMEA were extracted from EPARs (European Public Assessment Reports). Active substance, year of approval, anatomical, therapeutic and chemical (ATC) code, indication, orphan status, ages, and registrative clinical studies characteristics were assessed.
Results
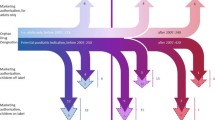
The percentage of authorised substances for paediatrics is 33.3%. This percentage decreased or increased when different subsets of medicines were considered [medicines for children under 2 years (23.4%), N-ATC code drugs (6%) and orphan drugs (46.4%)]. A total of 165 trials were included in the MA dossiers of 51 drugs at the time of approval, and additional 22 studies were added to the dossiers of 12 active substances submitted for paediatric variations. PK and Efficacy/Safety studies were performed for 32 (52%) active substances, while either one PK or one Efficacy/Safety study was carried out for 43 (69%) and 45 (73%) substances, respectively.
Conclusions
This report demonstrates that the total number of paediatric medicines approved by EMEA is stable over the 10-year period, while an increase in drugs to treat serious or orphan diseases has been observed. In addition, under the Centralised Procedure, a valuable number of paediatric trials have been submitted to support drug approval.
Similar content being viewed by others
Notes
TEDDY is a EU funded project aimed at favouring the development and the rationale use of drugs for the paediatric population.
the Label (labelling) is defined according to the DIRECTIVE 2001/83/EC (art.1) as the ‘information on the immediate or outer packaging’ while the ‘Package leaflet’ is ‘a leaflet containing information for the user which accompanies the medicinal product’ [5].
Orphan-like drugs are drugs intended for patients with rare diseases, authorised prior to introduction of the “Orphan” legislation and for whom EMEA provided support in the form of fee reductions for post-authorisation activities [6].
Vaccines are excluded by the analysis.
References
European Commission Enterprise Directorate General (2002) Better Medicines for Children. Proposed regulatory actions on paediatric medicinal products. Consultation document. Eudralex
Ceci A, Felisi M, Catapano M, Baiardi P, Cipollina L, Ravera S, Bagnulo S, Reggio S, Rondini G (2002) Medicines for children licensed by the European Agency for the Evaluation of Medicinal Products. Eur J Clin Pharmacol 58:495–500
European Parliament and Council Regulation 141/2000/EC, 16 December 1999 on Orphan Medicinal Products. Official Journal of the European Communities L018/1 22/01/2000
Baiardi P, Felisi M, Cipollina L, Lerro G, Carnelli V, Rondini G, Ceci A (2002) Availability of paediatric medicines in Italy. Quaderni di Pediatria 3:203–206
European Parliament and Council Directive 2001/83/EC, 6 November 2001 on the Community code relating to medicinal products for human use. Official Journal of the European Communities L311, P. 0067–0128, 28.11.2001
ICH (2000) Clinical Investigation of Medicinal Products in the Paediatric Population. ICH/Topic E11. Eudralex
ICH (1997) General considerations for clinical trials. ICH Topic E8 Guideline
EMEA (2001) Status Report on the implementation of the European Parliament Legislation on Orphan Medicinal Products EMEA/7381/01 (March 30th 2001)
Impicciatore P, Choonara I (1999) Status of new medicines approved by the European Medicines Evaluation Agency regarding paediatric use. Br J Clin Pharmacol 48:15–18
Koren G, Barzilay Z, Greenwald M (1986) Tenfold errors in administration of drugs doses: a neglected iatrogenic diseases in paediatrics. Paediatrics 77:848–849
Ghaleb MA, Barber N, Franklin BD, Wong ICK (2005) What constitutes a prescribing error in paediatrics? Qual Saf Health Care 14:352–357
‘t Jong GW, van der Linden PD, Bakker EM, van der Lely N, Eland IA, Stricker BH, van den Anker JN (2002) Unlicensed and off-label drug use in a paediatric ward of a general hospital in the Netherlands. Eur J Clin Pharmacol 58:293–297
Eurordis (2005) Medicines for children: better, more and faster. Eurordis position paper on the proposal for a regulation on medicinal products for paediatric use. Available at http://www.eurordis.org/IMG/pdf/eurordis_position_medicines_children_31jan05.pdf.
European Commission (EC) (2004) Proposal for “Regulation Of The European Parliament And Of The Council on medicinal products for paediatric use and amending Council Regulation (EEC) No 1768/92, Directive 2001/83/EC and Regulation (EC) No 726/2004. COM(2004)
Lantos JD (1999) The “inclusion benefit” in clinical trials. J Pediatr 134:130–131
Food and Drug Administration (1994) Specific requirements on content and format of labeling for human prescription drugs: revision of “pediatric use” subsection in the labeling. Fed Reg 59:64240–64250
Food and Drug Administration(1998) Regulations requiring manufacturers to assess the safety and effectiveness of new drugs and biological products in pediatric patients: final rule. Fed Reg 63:66632–66672
Apolone G, Joppi R, Bertele’ V, Garattini S (2005) Ten years of marketing approvals of anticancer drugs in Europe: regulatory policy and guidance documents need to find a balance between different pressures. Br J Cancer 93:504–509
Joppi R, Bertele V, Garattini S (2006) Orphan drug development is progressing too slowly. Br J Clin Pharmacol 61:355–360
Acknowledgements
The authors wish to thank Cristina Manfredi and Elisa Cattani for their technical and secretarial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is part of the Network of Excellence TEDDY (Task-Force in Europe for the Drug Development in the Young) supported by the EC-Sixth Framework Program (Contract n. 0005216 LSHB-CT-2005-005126).
Rights and permissions
About this article
Cite this article
Ceci, A., Felisi, M., Baiardi, P. et al. Medicines for children licensed by the European Medicines Agency (EMEA): the balance after 10 years. Eur J Clin Pharmacol 62, 947–952 (2006). https://doi.org/10.1007/s00228-006-0193-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0193-0