Abstract
Background
Mesalazine undergoes extensive metabolism by N-acetylation. While there is some evidence for an involvement of N-acetyltransferase (NAT) type 1, a potential role of NAT type 2 (NAT2) in vivo has not been tested.
Methods
In two studies in healthy young Caucasians, NAT2 phenotyping was carried out using a caffeine metabolic ratio in urine 4-6 h postdose. In study A, 1,000 mg mesalazine doses were given thrice daily for 5 days, and urine and blood samples were drawn during the last dosing interval. In study B, a 1,000 mg single dose was given, and samples were taken for 48 h postdose. Pharmacokinetics of mesalazine and N-acetylmesalazine (LC-MS/MS) were calculated by noncompartmental methods.
Results
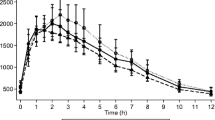
NAT2 phenotype could be allocated unequivocally in 21 slow and 5 rapid acetylators in study A, and in 9 slow and 8 rapid acetylators in study B. Geometric mean (CV%) values in study A for slow [rapid] acetylators were as follows: mesalazine AUC 11.1 µg/mL·h (51%) [12.0 µg/mL·h (52%)], N-acetylmesalazine AUC 27.7 µg/mL·h (32%) [30.5 µg/mL·h (27%)], mesalazine Ae 8.53% (89%) [9.03% (52%)], N-acetylmesalazine Ae 31.4% (46%) [32.2 (41%)]. Values in study B were as follows: mesalazine AUC 3.45 µg/mL·h (113%) [2.36 µg/mL·h (87%)], N-acetylmesalazine AUC 21.3 µg/mL·h (29%) [18.0 µg/mL·h (39%)], mesalazine Ae 0.2% (256%) [0.1% (359%)], N-acetylmesalazine Ae 30.9% (44%) [18.1% (84%)]. Higher AUC and Ae values for mesalazine in steady state study indicate saturation of mesalazine metabolism. Statistics provided no evidence for a true difference in mesalazine pharmacokinetics between slow and rapid acetylators, and no significant correlation between NAT2 activity and any mesalazine pharmacokinetic parameter was found.
Conclusion
NAT2 has no major role in human metabolism of mesalazine in vivo.




Similar content being viewed by others
References
Klotz U, Maier KE (1987) Pharmacology and pharmacokinetics of 5-aminosalicylic acid. Dig Dis Sci 32(12 Suppl):46S–50S
Estrada-Rodgers L, Levy GN, Weber WW (1998) Substrate selectivity of mouse N-acetyltransferases 1, 2, and 3 expressed in COS-1 cells. Drug Metab Dispos 26(5):502–505
Hein DW, Doll MA, Rustan TD, Gray K, Feng Y, Ferguson RJ et al (1993) Metabolic activation and deactivation of arylamine carcinogens by recombinant human NAT1 and polymorphic NAT2 acetyltransferases. Carcinogenesis 14(8):1633–1638
Windmill KF, Gaedigk A, Hall PM, Samaratunga H, Grant DM, McManus ME (2000) Localization of N-acetyltransferases NAT1 and NAT2 in human tissues. Toxicol Sci 54(1):19–29
Fischer C, Maier K, Stumpf E, von Gaisberg U, Klotz U (1983) Disposition of 5-aminosalicylic acid, the active metabolite of sulphasalazine, in man. Eur J Clin Pharmacol 25(4):511–515
Azad Khan AK, Nurazzaman M, Truelove SC (1983) The effect of the acetylator phenotype on the metabolism of sulphasalazine in man. J Med Genet 20(1):30–36
Bondesen S, Hegnhoj J, Larsen F, Hansen SH, Hansen CP, Rasmussen SN (1991) Pharmacokinetics of 5-aminosalicylic acid in man following administration of intravenous bolus and per os slow-release formulation. Dig Dis Sci 36(12):1735–1740
Allgayer H, Ahnfelt NO, Kruis W, Klotz U, Frank-Holmberg K, Soderberg HN et al (1989) Colonic N-acetylation of 5-aminosalicylic acid in inflammatory bowel disease. Gastroenterology 97(1):38–41
Ricart E, Taylor WR, Loftus EV, O’Kane D, Weinshilboum RM, Tremaine WJ et al (2002) N-acetyltransferase 1 and 2 genotypes do not predict response or toxicity to treatment with mesalamine and sulfasalazine in patients with ulcerative colitis. Am J Gastroenterol 97(7):1763–1768
Hausmann M, Paul G, Menzel K, Brunner-Ploss R, Falk W, Scholmerich J et al (2008) NAT1 genotypes do not predict response to mesalamine in patients with ulcerative colitis. Z Gastroenterol 46(3):259–265
Jetter A, Kinzig-Schippers M, Illauer M, Hermann R, Erb K, Borlak J et al (2004) Phenotyping of N-acetyltransferase type 2 by caffeine from uncontrolled dietary exposure. Eur J Clin Pharmacol 60(1):17–21
Kinzig-Schippers M, Tomalik-Scharte D, Jetter A, Scheidel B, Jakob V, Rodamer M et al (2005) Should we use N-acetyltransferase type 2 genotyping to personalize isoniazid doses? Antimicrob Agents Chemother 49(5):1733–1738
Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL et al (2001) Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev 10(12):1239–1248
Jetter A, Kinzig-Schippers M, Illauer M, Tomalik-Scharte D, Sörgel F, Fuhr U (2004) When should urine samples for NAT2 phenotyping with caffeine be collected? Clin Pharmacol Ther 75(2):P69
Fuhr U, Jetter A, Kirchheiner J (2007) Appropriate phenotyping procedures for drug metabolizing enzymes and transporters in humans and their simultaneous use in the “cocktail” approach. Clin Pharmacol Ther 81(2):270–283
Fuhr U, Rost KL, Engelhardt R, Sachs M, Liermann D, Belloc C et al (1996) Evaluation of caffeine as a test drug for CYP1A2, NAT2 and CYP2E1 phenotyping in man by in vivo versus in vitro correlations. Pharmacogenetics 6(2):159–176
Hickman D, Pope J, Patil SD, Fakis G, Smelt V, Stanley LA et al (1998) Expression of arylamine N-acetyltransferase in human intestine. Gut 42(3):402–409
Sandborn WJ, Hanauer SB (2003) Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol Ther 17(1):29–42
Hardy JG, Harvey WJ, Sparrow RA, Marshall GB, Steed KP, Macarios M et al (1993) Localization of drug release sites from an oral sustained-release formulation of 5-ASA (Pentasa) in the gastrointestinal tract using gamma scintigraphy. J Clin Pharmacol 33(8):712–718
Ireland A, Priddle JD, Jewell DP (1990) Acetylation of 5-aminosalicylic acid by isolated human colonic epithelial cells. Clin Sci (Lond) 78(1):105–111
Mahid SS, Colliver DW, Crawford NP, Martini BD, Doll MA, Hein DW et al (2007) Characterization of N-acetyltransferase 1 and 2 polymorphisms and haplotype analysis for inflammatory bowel disease and sporadic colorectal carcinoma. BMC Med Genet 8:28
Delomenie C, Fouix S, Longuemaux S, Brahimi N, Bizet C, Picard B et al (2001) Identification and functional characterization of arylamine N-acetyltransferases in eubacteria: evidence for highly selective acetylation of 5-aminosalicylic acid. J Bacteriol 183(11):3417–3427
van Hogezand RA, Kennis HM, van Schaik A, Koopman JP, van Hees PA, van Tongeren JH (1992) Bacterial acetylation of 5-aminosalicylic acid in faecal suspensions cultured under aerobic and anaerobic conditions. Eur J Clin Pharmacol 43(2):189–192
Walraven JM, Trent JO, Hein DW (2008) Structure-function analyses of single nucleotide polymorphisms in human N-acetyltransferase 1. Drug Metab Rev 40(1):169–184
Bruhn C, Brockmoller J, Cascorbi I, Roots I, Borchert HH (1999) Correlation between genotype and phenotype of the human arylamine N-acetyltransferase type 1 (NAT1). Biochem Pharmacol 58(11):1759–1764
Dilger K, Trenk D, Rossle M, Cap M, Zahringer A, Wacheck V et al (2007) A clinical trial on absorption and N-acetylation of oral and rectal mesalazine. Eur J Clin Invest 37(7):558–565
Muller AF, Stevens PE, McIntyre AS, Ellison H, Logan RF (2005) Experience of 5-aminosalicylate nephrotoxicity in the United Kingdom. Aliment Pharmacol Ther 21(10):1217–1224
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lück, H., Kinzig, M., Jetter, A. et al. Mesalazine pharmacokinetics and NAT2 phenotype. Eur J Clin Pharmacol 65, 47–54 (2009). https://doi.org/10.1007/s00228-008-0550-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0550-2