Abstract
Purpose
Since many drug targets and metabolizing enzymes are developmentally regulated, we investigated a potential comparable regulation of inosine 5’-monophosphate dehydrogenase (IMPDH) activity that has recently been advocated as a pharmacodynamic biomarker of mycophenolic acid (MPA) effects in the paediatric population. Since the field of pharmacodynamic monitoring of MPA is evolving, we also analyzed the response of IMPDH activity on MPA in children vs adolescents after renal transplantation.
Methods
We analyzed IMPDH activity in peripheral blood mononuclear cells (PBMCs) in 79 healthy children aged 2.0–17.9 years in comparison to 106 healthy adults. Pharmacokinetic/pharmacodynamic profiles of MPA and IMPDH over 6 or 12 h after mycophenolate mofetil dosing were performed in 17 paediatric renal transplant recipients. IMPDH activity was measured by HPLC and normalized to the adenosine monophosphate (AMP) content of the cells, MPA plasma concentrations were measured by HPLC.
Results
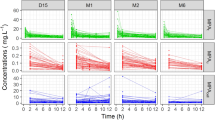
Inosine 5’-monophosphate dehydrogenase activity displayed a high inter-individual variability (coefficient of variation 40.2%) throughout the entire age range studied. Median IMPDH did not differ significantly in healthy pre-school children (82 [range, 42–184] μmol/s/mol AMP), school-age children (61 [30–153]), adolescents (83 [43–154]) and healthy adults (83 [26–215]). Similar to adults, IMPDH activity in children and adolescents was inversely correlated with MPA plasma concentration.
Conclusions
In conclusion, our data do not show a pronounced developmental regulation of IMPDH activity in PBMCs in the paediatric population and there is a comparable inhibition of IMPDH activity by MPA in children and adolescents after renal transplantation.




Similar content being viewed by others
Abbreviations
- A:
-
IMPDH activity
- AEC:
-
Area under the enzyme activity–time curve
- AIC:
-
Akaike information criterion
- Alow :
-
Maximal possible IMPDH inhibition
- Amin :
-
Minimum IMPDH activity
- AMP:
-
Adenosine monophosphate
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the concentration–time curve
- BSA:
-
Body surface area
- BMI SDS:
-
Body mass index standard deviation score
- C:
-
MPA concentration
- CL/F:
-
Apparent drug clearance
- Cmax :
-
Maximum MPA concentration
- D:
-
Administered MPA content
- ESRD:
-
End-stage renal disease
- Freq:
-
Frequency
- H:
-
Sigmoidicity parameter
- HPLC:
-
High-performance liquid chromatography
- IMPDH:
-
Inosine 5’-monophosphate dehydrogenase
- MPA:
-
Mycophenolic acid
- MMF:
-
Mycophenolate mofetil
- PBMCs:
-
Peripheral blood mononuclear cells
- PD:
-
Pharmacodynamic
- PK:
-
Pharmacokinetic
- RTx:
-
Renal transplantation
- SD:
-
Standard deviation
- SNP:
-
Single nucleotide polymorphism
- tAmin :
-
Time to minimum IMPDH activity
- tCmax :
-
Time of maximum MPA concentration in a dosing interval
- XMP:
-
Xanthosine 5’-monophosphate
References
Allison AC, Eugui EM (1996) Purine metabolism and immunosuppressive effects of mycophenolate mofetil. Clin Transpl 10:77–84
Tönshoff B, David-Neto E, Ettenger R, Filler G, van Gelder T, Goebel J, Kuypers DRJ, Tsai E, Vinks AA, Weber LT, Zimmerhackl LB (2011) Pediatric aspects of therapeutic drug monitoring of mycophenolic acid in renal transplantation. Transplant Rev 25:78–89
Bunchman T, Navarro M, Broyer M, Sherbotie J, Chavers B, Tönshoff B et al (2001) The use of mycophenolate mofetil suspension in pediatric renal allograft recipients. Pediatr Nephrol 16:978–984
Höcker B, van Gelder T, Martin-Govantes J, Machado P, Tedesco H, Rubik J et al (2011) Comparison of MMF efficacy and safety in paediatric vs. adult renal transplantation: subgroup analysis of the randomized, multicentre FDCC trial. Nephrol Dial Transplant 26:1073–1079
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167
Takahashi H, Ishikawa S, Nomoto S, Nishigaki Y, Ando F, Kashima T et al (2000) Developmental changes in pharmacokinetics and pharmacodynamics of warfarin enantiomers in Japanese children. Clin Pharmacol Ther 68:541–555
Marshall JD, Kearns GL (1999) Developmental pharmacodynamics of cyclosporine. Clin Pharmacol Ther 66:66–75
Marshall J, Rodarte A, Blumer J, Khoo KC, Akbari B, Kearns GL (2000) Pediatric pharmacodynamics of midazolam oral syrup. J Clin Pharmacol 40:578–589
de Wildt SN, Kearns GL, Sie HD, Hop WCJ, van den Anker JN (2003) Pharmacodynamics of intravenous and oral midazolam in preterm infants. Clin Drug Invest 23:27–38
Brouwer C, Vermunt-de Koning DG, Trueworthy RC, Ter Riet PG, Duley JA, Trijbels FJ et al (2006) Monitoring of inosine monophosphate dehydrogenase activity in mononuclear cells of children with acute lymphoblastic leukemia: enzymological and clinical aspects. Pediatr Blood Cancer 46:434–438
Fukuda T, Goebel J, Thogersen H, Maseck D, Cox S, Logan B et al (2011) Inosine monophosphate dehydrogenase (IMPDH) activity as a pharmacodynamic biomarker of mycophenolic acid effects in pediatric kidney transplant recipients. J Clin Pharmacol 51:309–320
Budde K, Glander P, Braun KP, Böhler T, Waiser J, Fritsche L et al (2001) Pharmacodynamic monitoring of mycophenolate mofetil in renal allograft recipients. Transplant Proc 33:3313–3315
Budde K, Bauer S, Hambach P, Hahn U, Röblitz H, Mai I et al (2007) Pharmacokinetic and pharmacodynamic comparison of enteric-coated mycophenolate sodium and mycophenolate mofetil in maintenance renal transplant patients. Am J Transplant 7:888–898
Chiarelli LR, Molinaro M, Libetta C, Tinelli C, Cosmai L, Valentini G et al (2010) Inosine monophosphate dehydrogenase variability in renal transplant patients on long-term mycophenolate mofetil therapy. Br J Clin Pharmacol 69:38–50
Wang J, Zeevi A, Webber S, Girnita DM, Addonizio L, Selby R et al (2007) A novel variant L263F in human inosine 5'-monophosphate dehydrogenase 2 is associated with diminished enzyme activity. Pharmacogenet Genomics 17:283–290
Wang J, Yang JW, Zeevi A, Webber SA, Girnita DM, Selby R et al (2008) IMPDH1 gene polymorphisms and association with acute rejection in renal transplant patients. Clin Pharmacol Ther 83:711–717
Grinyó J, Vanrenterghem Y, Nashan B, Vincenti F, Ekberg H, Lindpaintner K et al (2008) Association of four DNA polymorphisms with acute rejection after kidney transplantation. Transpl Int 21:879–891
Sombogaard F, van Schaik RH, Mathot RA, Budde K, van der Werf M, Vulto AG et al (2009) Interpatient variability in IMPDH activity in MMF-treated renal transplant patients is correlated with IMPDH type II 3757 T > C polymorphism. Pharmacogenet Genomics 19:626–634
Gensburger O, Van Schaik RH, Picard N, Le Meur Y, Rousseau A, Woillard JB et al (2010) Polymorphisms in type I and II inosine monophosphate dehydrogenase genes and association with clinical outcome in patients on mycophenolate mofetil. Pharmacogenet Genomics 20:537–543
North American Pediatric Renal Trials and Collaborative Studies, Annual Transplant Report 2010. http://www.emmes.com/study/ped/annlrept/2010_Report.pdf. Accessed 26 July 2011
Brandhorst G, Streit F, Goetze S, Oellerich M, Armstrong VW (2006) Quantification by liquid chromatography tandem mass spectrometry of mycophenolic acid and its phenol and acyl glucuronide metabolites. Clin Chem 52:1962–1964
Glander P, Sombogaard F, Budde K, van Gelder T, Hambach P, Liefeldt L et al (2009) Improved assay for the nonradioactive determination of inosine 5’-monophosphate dehydrogenase activity in peripheral blood mononuclear cells. Ther Drug Monit 31:351–359
Akaike H (1973) Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Second International Symposium on Information Theory. Akademiai Kiado, Budapest, pp 267–281
Guo SW, Thompson EA (1992) Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics 48:361–372
Liu K, Muse SV (2005) PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129
Lin DY, Zeng D, Millikan R (2005) Maximum likelihood estimation of haplotype effects and haplotype-environment interactions in association studies. Genet Epidemiol 29:299–312
Langman LJ, LeGatt DF, Halloran PF, Yatscoff RW (1996) Pharmacodynamic assessment of mycophenolic acid-induced immunosuppression in renal transplant recipients. Transplantation 62:666–672
Glander P, Hambach P, Braun KP, Fritsche L, Waiser J, Mai I et al (2003) Effect of mycophenolate mofetil on IMP dehydrogenase after the first dose and after long-term treatment in renal transplant recipients. Int J Clin Pharmacol Ther 41:470–476
Weber LT, Lamersdorf T, Shipkova M, Niedmann PD, Wiesel M, Zimmerhackl LB et al (1999) Area under the plasma concentration-time curve for total, but not for free, mycophenolic acid increases in the stable phase after renal transplantation: a longitudinal study in pediatric patients. Ther Drug Monit 21:498–506
Nowak J, Shaw LM (1995) Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin Chem 41:1011–1017
Weber LT, Shipkova M, Armstrong VW, Wagner N, Schütz E, Mehls O et al (2002) The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic acid in pediatric renal transplant recipients; a report of the German study group on mycophenolate mofetil therapy. J Am Soc Nephrol 13:759–768
Glander P, Braun KP, Hambach P, Bauer S, Mai I, Roots I et al (2001) Non-radioactive determination of inosine 5`-monophosphate dehydrogenase (IMPDH) in peripheral mononuclear cells. Clin Biochem 34:543–549
Budde K, Glander P, Krämer BK, Fischer W, Hoffmann U, Bauer S et al (2007) Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in maintenance renal transplant recipients receiving tacrolimus: clinical, pharmacokinetic, and pharmacodynamic outcomes. Transplantation 83:417–424
Weimert NA, DeRotte M, Alloway RR, Woodle ES, Vinks AA (2007) Monitoring of inosine monophosphate dehydrogenase activity as a biomarker for mycophenolic acid effect: potential clinical implications. Ther Drug Monit 29:141–149
Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT et al (2005) Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 353:2219–2228
Glander P, Hambach P, Braun KP, Fritsche L, Giessing M, Mai I et al (2004) Pre-transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant 4:2045–2051
Sombogaard F, Mathot R, Ie HY, Glander P, Weimar W, van Gelder T (2008) IMPDH activity on day 6 after kidney transplantation is significantly related to the risk of acute rejection in MMF treated patients. Am J Transplant 8 [Suppl]:252
Cole TJ (1990) The LMS method for constructing normalized growth standards. Eur J Clin Nutr 44:45–60
Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317:1098
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA et al (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Myara I, Lahiani F, Cosson C, Duboust A, Moatti N (1989) Estimated creatinine clearance by the formula of Gault and Cockcroft in renal transplantation. Nephron 51:426–427
Acknowledgements
We gratefully acknowledge the expert technical assistance of Sandra Hartung and Ulrike Hügel.
Funding source
This study was supported by the Peter-Stiftung für die Nierenwissenschaft (scientific foundation to promote kidney research, particularly in children). The manuscript was not prepared or funded by a commercial organisation.
Conflicts of interest
Lutz T. Weber and Burkhard Tönshoff have received research grants from Roche Pharma AG and Novartis Pharma GmbH. Klemens Budde has received research grants and honoraria from Roche Pharma AG and Novartis Pharma GmbH.
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Tönshoff and L. T. Weber contributed equally to this work
V. W. Armstrong: Date of death: 27 February 2010
Rights and permissions
About this article
Cite this article
Rother, A., Glander, P., Vitt, E. et al. Inosine monophosphate dehydrogenase activity in paediatrics: age-related regulation and response to mycophenolic acid. Eur J Clin Pharmacol 68, 913–922 (2012). https://doi.org/10.1007/s00228-011-1203-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1203-4