Abstract
Purpose
To compare the pharmacokinetics and pharmacodynamics of tolvaptan in Caucasian and Japanese healthy male subjects under fasting and non-fasting conditions.
Methods
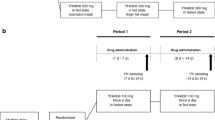
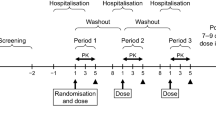
This was a single-center, parallel-group, randomized, open-label, three-period crossover trial of single oral doses of tolvaptan 30 mg under fasting and non-fasting [a high-fat, high-calorie meal (HFM) or Japanese standard meal] conditions in 25 healthy male Caucasian subjects and 24 healthy male Japanese subjects. Pharmacodynamic endpoints were urine volume and fluid balance for 0 to 24 h postdose.
Results
In the fasted state, the plasma tolvaptan Cmax and AUC∞ geometric mean ratios (90 % confidence interval) were 1.105 (0.845–1.444) and 1.145 (0.843–1.554) for Japanese compared to Caucasian subjects. A HFM increased the Cmax and AUC∞ values by about 1.15-fold in both Japanese and Caucasian subjects.. Twenty-four-hour urine volumes paralleled pharmacokinetic changes, but the increases were not clinically significant. Fluid balance in the Japanese men was 1.4- to 2.0-fold more negative than that in the Caucasian men.
Conclusion
Tolvaptan pharmacokinetics is not clinically significantly affected by race. Body weight is a factor that affects exposure. Tolvaptan can be administered with or without food.



Similar content being viewed by others
References
Yasuda SU, Zhang L, Huang SM (2008) The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther 84(3):417–423. doi:10.1038/clpt.2008.141
Fukunaga S, Kusama M, Arnold FL, Ono S (2011) Ethnic differences in pharmacokinetics in new drug applications and approved doses in Japan. J Clin Pharmacol 51(8):1237–1240. doi:10.1177/0091270010381500
Kim K, Johnson JA, Derendorf H (2004) Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol 44(10):1083–1105. doi:10.1177/0091270004268128
Johnson JA (2000) Predictability of the effects of race or ethnicity on pharmacokinetics of drugs. Int J Clin Pharmacol Ther 38(2):53–60
Chen ML (2006) Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 45(10):957–964
Uyama Y, Shibata T, Nagai N, Hanaoka H, Toyoshima S, Mori K (2005) Successful bridging strategy based on ICH E5 guideline for drugs approved in Japan. Clin Pharmacol Ther 78(2):102–113. doi:10.1016/j.clpt.2005.04.001
Ichimaru K, Toyoshima S, Uyama Y (2010) Effective global drug development strategy for obtaining regulatory approval in Japan in the context of ethnicity-related drug response factors. Clin Pharmacol Ther 87(3):362–366. doi:10.1038/clpt.2009.285
Gu CH, Li H, Levons J, Lentz K, Gandhi RB, Raghavan K, Smith RL (2007) Predicting effect of food on extent of drug absorption based on physicochemical properties. Pharm Res 24(6):1118–1130. doi:10.1007/s11095-007-9236-1
Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, Yamada Y, Tsujimae K, Aoyama M, Kotosai K, Ogawa H, Yamashita H, Kondo K, Tominaga M, Tsujimoto G, Mori T (1998) OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 287(3):860–867
King LS, Agre P (1996) Pathophysiology of the aquaporin water channels. Annu Rev Physiol 58:619–648. doi:10.1146/annurev.ph.58.030196.003155
Shoaf SE, Wang Z, Bricmont P, Mallikaarjun S (2007) Pharmacokinetics, pharmacodynamics, and safety of tolvaptan, a nonpeptide AVP antagonist, during ascending single-dose studies in healthy subjects. J Clin Pharmacol 47(12):1498–1507. doi:10.1177/0091270007307877
Fried LF, Palevsky PM (1997) Hyponatremia and hypernatremia. Med Clin North Am 81(3):585–609
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2002) Guidance for industry: food-effect bioavailability and fed bioequivalence studies. CDER, Silver Spring, pp 1–12
Jones B, Kenward MG (1989) Design and analysis of cross-over trials. Chapman and Hall, London
Jusko WJ (1992) Guidelines for collection and analysis of pharmacokinetic data. In: Evans WE, Jusko WJ, Schentag JJ (eds) Applied pharmacokinetics: principles of therapeutic drug monitoring, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, p 1202
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2002) Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. CDER, Silver Spring, pp 1–23
Dangi YS, Soni ML, Namdeo KP (2010) Highly variable drugs: Bioequivalence requirement and regulatory perspectives. J Curr Pharm Res 3:24–8
Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C (2006) Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355(20):2099–2112. doi:10.1056/NEJMoa065181
Acknowledgments
We would like to thank Osamu Sato of Clinical Research & Development, Otsuka Pharmaceutical Co, Japan, for his help with design of the trial. The editorial support of Upside Endeavors LLC in the preparation of this manuscript was funded by Otsuka America Pharmaceutical, Inc.
Declaration of interest
The authors are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. (OPDC) or Otsuka Pharmaceutical Co, Japan.
Role of funding source
OPDC provided all funding for the trial.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shoaf, S.E., Kim, S.R., Bricmont, P. et al. Pharmacokinetics and pharmacodynamics of single-dose oral tolvaptan in fasted and non-fasted states in healthy Caucasian and Japanese male subjects. Eur J Clin Pharmacol 68, 1595–1603 (2012). https://doi.org/10.1007/s00228-012-1295-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1295-5