Abstract
Purpose
There is a lack of knowledge about drug-related problems (DRPs) among pregnant and lactating women. The aim of this study was to determine the extent and type of DRPs among pregnant and lactating women in the maternity ward at two Norwegian hospitals. We also aimed to investigate which drugs were involved in the identified DRPs, and the outcome of solving the DRPs.
Methods
Patient-reported treatment reviews were performed to assess the prevalence and type of DRPs among women at the two maternity wards.
Results
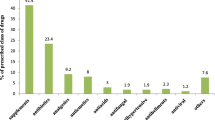
In all, 212 women were included in the study, of which 89 (42 %) had experienced at least one DRP (105 DRPs in total). “Need for additional drug” (49 cases, 46.7 %) was the most frequent. The most frequent drug group involved in DRPs was drugs acting on the respiratory system, and the most common intervention was raising awareness/providing confidence/giving information during the patient-reported treatment review.
Conclusions
Over four out of ten women in the maternity wards have DRPs, and many have questions about drug use during pregnancy and lactation. Many of the DRPs could probably be avoided by providing patient-reported treatment reviews to pregnant women as a part of antenatal care. Multidisciplinary collaboration including physicians, midwifes, and pharmacists in antenatal care and in maternity ward could possibly prevent DRPs and thereby promote patient safety for pregnant and lactating women.

Similar content being viewed by others
References
Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S (2007) Drug-related problems in hospitals: a review of the recent literature. Drug Saf 30(5):379–407
Lisby M, Nielsen LP, Mainz J (2005) Errors in the medication process: frequency, type, and potential clinical consequences. Int J Qual Health Care 17(1):15–22
Blix HS, Viktil KK, Reikvam A, et al. (2004) The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. Eur J Clin Pharmacol 60(9):651–658
Pharmaceutical Care Network Europe (PCNE) (2010) Classification for Drug related problems. The PCNE Classification V 6.2. http://www.pcne.org/upload/files/11_PCNE_classification_V6-2.pdf. [Accessed 29.01.2016]
Viktil KK, Blix HS (2008) The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol Toxicol 102(3):275–280
Basger BJ, Moles RJ, Chen TF (2014) Application of drug-related problem (DRP) classification systems: a review of the literature. Eur J Clin Pharmacol 70(7):799–815
Bates DW (1996) Medication errors. How common are they and what can be done to prevent them? Drug Saf 15(5):303–310
Mannesse CK, Derkx FH, de Ridder MA, AJ Man in’t Veld AJ, der Cammen TJ v (2000) Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing 29(1):35–39
Ebbesen J, Buajordet I, Erikssen J, et al. (2001) Drug-related deaths in a department of internal medicine. Arch Intern Med 161(19):2317–2323
Ernst FR, Grizzle AJ (2001) Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash) 41(2):192–199
Thompson R, Whennan L, Liang J, Alderman C, Grzeskowiak LE (2015) Investigating the Frequency and Nature of Medication-Related Problems in the Women’s Health Unit of an Australian Tertiary Teaching Hospital. Ann Pharmacother 49(7):770–776
Fernandez-Llamazares CM, Calleja-Hernandez MA, Manrique-Rodriguez S, Perez-Sanz C, Duran-Garcia E, Sanjurjo-Saez M (2012) Prescribing errors intercepted by clinical pharmacists in paediatrics and obstetrics in a tertiary hospital in Spain. Eur J Clin Pharmacol 68(9):1339–1345
Kandil M, Sayyed T, Emarh M, Ellakwa H, Masood A (2012) Medication errors in the obstetrics emergency ward in a low resource setting. J Matern Fetal Neonatal Med 25(8):1379–1382
Kfuri TA, Morlock L, Hicks RW, Shore AD (2008) Medication errors in obstetrics. Clin Perinatol 35(1):101–117 viii-ix
Beyea SC, Kobokovich LJ, Becker SC, Hicks RW (2004) Medication errors in the LDRP. AWHONN Lifelines 8(2):130–140
Little JA, Velazquez MB, Rayburn WF (2003) Reported medication errors in obstetric inpatients in 1 hospital. J Reprod Med 48(10):818–820
Lesar TS, Briceland LL, Delcoure K, Parmalee JC, Masta-Gornic V, Pohl H (1990) Medication prescribing errors in a teaching hospital. JAMA 263(17):2329–2334
Freyer AM (2008) Drug-prescribing challenges during pregnancy. Obstet, Gynaecol Reprod Med 18(7):180–186
Frederiksen MC (2001) Physiologic changes in pregnancy and their effect on drug disposition. Semin Perinatol 25(3):120–123
Koren G, Pastuszak A, Ito S (1998) Drugs in pregnancy. N Engl J Med 338(16):1128–1137
Nordeng H, Ystrom E, Einarson A (2010) Perception of risk regarding the use of medications and other exposures during pregnancy. Eur J Clin Pharmacol 66(2):207–214
Matsui D (2012) Adherence with drug therapy in pregnancy. Obstet Gynecol Int :Article ID 796590.
Zabihi S, Loeken MR (2010) Understanding diabetic teratogenesis: where are we now and where are we going? Birth Defects Res A Clin Mol Teratol 88(10):779–790
Vajda FJ, Lander CM, Hitchcock A, et al. (2007) Changing Australian prescribing patterns for antiepileptic drugs in pregnancy and their possible consequences. J Clin Neurosci 14(7):611–617
Nordeng H (2016) Drug utilisation studies in pregnancy.In: Elseviers M, Wettermark B, Almarsdottir AB, et al., editors. In: Drug utilization research: methods and applications. John Wiley & Sons, Oxford
Daw JR, Hanley GE, Greyson DL, Morgan SG (2011) Prescription drug use during pregnancy in developed countries: a systematic review. Pharmacoepidemiol Drug Saf 20(9):895–902
ATC/DDD index (2015) WHO Collaborating Centre for Drug Statistics Methodology. http://www.whocc.no/atc_ddd_index/. Accessed 17 March 2015
Ruths S, Viktil KK, Blix HS (2007) Classification of drug-related problems. Tidsskr Nor Laegeforen 127(23):3073–3076
Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S (2011) Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 205(1):51 e1–51 e8
Nordeng H, Bayne K, Havnen GC, Paulsen BS (2011) Use of herbal drugs during pregnancy among 600 Norwegian women in relation to concurrent use of conventional drugs and pregnancy outcome. Complement Ther Clin Pract 17(3):147–151
Lupattelli A, Spigset O, Twigg MJ, et al. (2014) Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open 4(2):e004365
Thorpe PG, Gilboa SM, Hernandez-Diaz S, et al. (2013) Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf 22(9):1013–1018
Hardy JR, Leaderer BP, Holford TR, Hall GC, Bracken MB (2006) Safety of medications prescribed before and during early pregnancy in a cohort of 81,975 mothers from the UK General Practice Research Database. Pharmacoepidemiol Drug Saf 15(8):555–564
Autret-Leca E, Deligne J, Leve J, Caille A, Cissoko H, Jonville-Bera AP (2011) Drug exposure during the periconceptional period: a study of 1793 women. Paediatr Drugs 13(5):317–324
Schirm E, Schwagermann MP, Tobi H, de Jong-van den Berg LT (2004) Drug use during breastfeeding. A survey from the Netherlands. Eur J Clin Nutr 58(2):386–390
Bergkvist Christensen A, Holmbjer L, Midlov P, et al. (2011) The process of identifying, solving and preventing drug related problems in the LIMM-study. Int J Clin Pharm 33(6):1010–1018
Willoch K, Blix HS, Pedersen-Bjergaard AM, Eek AK, Reikvam A (2012) Handling drug-related problems in rehabilitation patients: a randomized study. Int J Clin Pharm 34(2):382–388
Halvorsen KH, Ruths S, Granas AG, Viktil KK (2010) Multidisciplinary intervention to identify and resolve drug-related problems in Norwegian nursing homes. Scand J Prim Health Care 28(2):82–88
Blix HS, Viktil KK, Moger TA, Reikvam A (2006) Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm World Sci 28(3):152–158
Petersen I, McCrea RL, Lupattelli A, Nordeng H (2015) Women’s perception of risks of adverse fetal pregnancy outcomes: a large-scale multinational survey. BMJ Open 5(6):e007390
Marasinghe KM (2015) Computerised clinical decision support systems to improve medication safety in long-term care homes: a systematic review. BMJ Open 5(5):e006539
Lau B, Overby CL, Wirtz HS, Devine EB (2013) The association between use of a clinical decision support tool and adherence to monitoring for medication-laboratory guidelines in the ambulatory setting. Appl Clin Inform 4(4):476–498
Fritz D, Ceschi A, Curkovic I, et al. (2012) Comparative evaluation of three clinical decision support systems: prospective screening for medication errors in 100 medical inpatients. Eur J Clin Pharmacol 68(8):1209–1219
Loudon H, Nentin F, Silverman ME (2015) Using clinical decision support as a means of implementing a universal postpartum depression screening program. Arch Womens Ment Health.
Sarangarm P, Young B, Rayburn W, et al. (2012) Agreement between self-report and prescription data in medical records for pregnant women. Birth Defects Res A Clin Mol Teratol 94(3):153–161
van Gelder MM, van Rooij IA, de Walle HE, Roeleveld N, Bakker MK (2013) Maternal recall of prescription medication use during pregnancy using a paper-based questionnaire: a validation study in the Netherlands. Drug Saf 36(1):43–54
Acknowledgments
The authors would like to thank the involvement of staff within the maternity wards at Drammen and Ullevål Hospitals for their support and positive contributions toward this project. They are also grateful to all the participating women who took part in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Regional Ethics Committee in South-East Norway and the local ethics committees at the hospitals.
Conflict of interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
ESM 1
(DOCX 32.5 kb)
Rights and permissions
About this article
Cite this article
Smedberg, J., Bråthen, M., Waka, M.S. et al. Medication use and drug-related problems among women at maternity wards—a cross-sectional study from two Norwegian hospitals. Eur J Clin Pharmacol 72, 849–857 (2016). https://doi.org/10.1007/s00228-016-2042-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2042-0